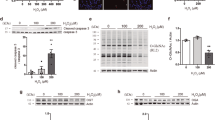
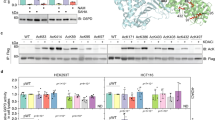
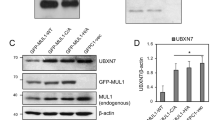
Abstract
Besides its role in glycolysis, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) initiates a cell death cascade1,2,3,4,5,6,7,8,9. Diverse apoptotic stimuli activate inducible nitric oxide synthase (iNOS) or neuronal NOS (nNOS), with the generated nitric oxide (NO) S-nitrosylating GAPDH, abolishing its catalytic activity and conferring on it the ability to bind to Siah1, an E3-ubiquitin-ligase with a nuclear localization signal (NLS). The GAPDH–Siah1 protein complex, in turn, translocates to the nucleus and mediates cell death; these processes are blocked by procedures that interfere with GAPDH–Siah1 binding. Nuclear events induced by GAPDH to kill cells have been obscure. Here we show that nuclear GAPDH is acetylated at Lys 160 by the acetyltransferase p300/CREB binding protein (CBP) through direct protein interaction, which in turn stimulates the acetylation and catalytic activity of p300/CBP. Consequently, downstream targets of p300/CBP, such as p53 (Refs 10,11,12,13,14,15), are activated and cause cell death. A dominant-negative mutant GAPDH with the substitution of Lys 160 to Arg (GAPDH-K160R) prevents activation of p300/CBP, blocks induction of apoptotic genes and decreases cell death. Our findings reveal a pathway in which NO-induced nuclear GAPDH mediates cell death through p300/CBP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hara, M. R. et al. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nature Cell Biol. 7, 665–674 (2005).
Chuang, D. M., Hough, C. & Senatorov, V. V. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu. Rev. Pharmacol. Toxicol. 45, 269–290 (2005).
Hara, M. R., Cascio, M. B. & Sawa, A. GAPDH as a sensor of NO stress. Biochim. Biophys. Acta 1762, 502–509 (2006).
Sirover, M. A. New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta 1432, 159–184 (1999).
Ishitani, R. et al. Evidence that glyceraldehyde-3-phosphate dehydrogenase is involved in age-induced apoptosis in mature cerebellar neurons in culture. J. Neurochem. 66, 928–935 (1996).
Sawa, A., Khan, A. A., Hester, L. D. & Snyder, S. H. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc. Natl Acad. Sci. USA 94, 11669–11674 (1997).
Carlile, G. W. et al. Reduced apoptosis after nerve growth factor and serum withdrawal: conversion of tetrameric glyceraldehyde-3-phosphate dehydrogenase to a dimer. Mol. Pharmacol. 57, 2–12 (2000).
Chen, R. W. et al. Involvement of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and p53 in neuronal apoptosis: evidence that GAPDH is upregulated by p53. J. Neurosci. 19, 9654–9662 (1999).
Waldmeier, P. C., Boulton, A. A., Cools, A. R., Kato, A. C. & Tatton, W. G. Neurorescuing effects of the GAPDH ligand CGP 3466B. J. Neural. Transm. Suppl. 60, 197–214 (2000).
Lill, N. L., Grossman, S. R., Ginsberg, D., DeCaprio, J. & Livingston, D. M. Binding and modulation of p53 by p300/CBP coactivators. Nature 387, 823–827 (1997).
Avantaggiati, M. L. et al. Recruitment of p300/CBP in p53-dependent signal pathways. Cell 89, 1175–1184 (1997).
Gu, W. & Roeder, R. G. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90, 595–606 (1997).
Chipuk, J. E., Bouchier-Hayes, L., Kuwana, T., Newmeyer, D. D. & Green, D. R. PUMA couples the nuclear and cytoplasmic proapoptotic function of p53. Science 309, 1732–1735 (2005).
Villunger, A. et al. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science 302, 1036–1038 (2003).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Ogryzko, V. V., Schiltz, R. L., Russanova, V., Howard, B. H. & Nakatani, Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87, 953–959 (1996).
Chan, H. M. & La Thangue, N. B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 114, 2363–2373 (2001).
Hara, M. R. et al. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc. Natl Acad. Sci. USA 103, 3887–3889 (2006).
Chen, L. F. & Greene, W. C. Regulation of distinct biological activities of the NF-κB transcription factor complex by acetylation. J. Mol. Med. 81, 549–557 (2003).
Thompson, P. R. et al. Regulation of the p300 HAT domain via a novel activation loop. Nature Struct. Mol. Biol. 11, 308–315 (2004).
Messmer, U. K., Ankarcrona, M., Nicotera, P. & Brune, B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 355, 23–26 (1994).
Messmer, U. K. & Brune, B. Nitric oxide-induced apoptosis: p53-dependent and p53-independent signalling pathways. Biochem. J. 319 (Pt 1), 299–305 (1996).
Yin, Y. et al. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature 391, 707–710 (1998).
Nowak, D. E., Tian, B. & Brasier, A. R. Two-step cross-linking method for identification of NF-κB gene network by chromatin immunoprecipitation. Biotechniques 39, 715–725 (2005).
Hess, D. T., Matsumoto, A., Kim, S. O., Marshall, H. E. & Stamler, J. S. Protein S-nitrosylation: purview and parameters. Nature Rev. Mol. Cell Biol. 6, 150–166 (2005).
Li, C. Q. et al. Apoptotic signaling pathways induced by nitric oxide in human lymphoblastoid cells expressing wild-type or mutant p53. Cancer Res. 64, 3022–3029 (2004).
Colell, A. et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell 129, 983–997 (2007).
Giordano, A. & Avantaggiati, M. L. p300 and CBP: partners for life and death. J. Cell Physiol. 181, 218–230 (1999).
Yao, T. P. et al. Gene dosage-dependent embryonic development and proliferation defects in mice lacking the transcriptional integrator p300. Cell 93, 361–372 (1998).
Kawasaki, H. et al. Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature 393, 284–289 (1998).
Kung, A. L. et al. Gene dose-dependent control of hematopoiesis and hematologic tumor suppression by CBP. Genes Dev. 14, 272–277 (2000).
Oike, Y. et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum. Mol. Genet. 8, 387–396 (1999).
Giles, R. H., Peters, D. J. & Breuning, M. H. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 14, 178–183 (1998).
Steffan, J. S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Turnell, A. S. et al. The APC/C and CBP/p300 cooperate to regulate transcription and cell-cycle progression. Nature 438, 690–695 (2005).
Dhakshinamoorthy, S. et al. Protein/DNA arrays identify nitric oxide-regulated cis-element and trans-factor activities some of which govern neuroblastoma cell viability. Nucleic Acids Res. 35, 5439–5451 (2007).
Koutsodontis, G., Vasilaki, E., Chou, W. C., Papakosta, P. & Kardassis, D. Physical and functional interactions between members of the tumour suppressor p53 and the Sp families of transcription factors: importance for the regulation of genes involved in cell-cycle arrest and apoptosis. Biochem. J. 389, 443–455 (2005).
Frade, J. M., Rodriguez-Tebar, A. & Barde, Y. A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature 383, 166–168 (1996).
Acknowledgements
This work was supported by USPHS grants MH-069853 (A.S.); DA-00266, Research Scientist Award DA-00074 (S.H.S); NS-48206, NS-38377, DA-00226 (T.M.D, V.L.D) and grants from Stanley, NARSAD and S-R foundations (A.S.). We thank Yukiko L. Lema for preparing the figures and organizing the manuscript. We appreciate technical assistance provided by A. Kamiya.
Author information
Authors and Affiliations
Contributions
Ni.S. and M.R.H. were primarily responsible for experimental design and work, data analysis and preparation of figures, and helped to write the manuscript; M.K., M.C., B.-I.B., Ne.S. and B.T. contributed to data acquisition and analysis; T.D. and V.D. helped with the data analysis, provided technical assistance and material support; S.H.S and A.S. supervised the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3, S4 and S5 (PDF 993 kb)
Rights and permissions
About this article
Cite this article
Sen, N., Hara, M., Kornberg, M. et al. Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat Cell Biol 10, 866–873 (2008). https://doi.org/10.1038/ncb1747
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1747
This article is cited by
-
A nuclear-located glyceraldehyde-3-phosphate dehydrogenase affects salt stress response processes in Arabidopsis thaliana as a senescence component
Journal of Plant Biochemistry and Biotechnology (2024)
-
Targeting protein modifications in metabolic diseases: molecular mechanisms and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Regulation of tumor metabolism by post translational modifications on metabolic enzymes
Cancer Gene Therapy (2023)
-
Classical epithelial-mesenchymal transition (EMT) and alternative cell death process-driven blebbishield metastatic-witch (BMW) pathways to cancer metastasis
Signal Transduction and Targeted Therapy (2022)
-
Proteins moonlighting in tumor metabolism and epigenetics
Frontiers of Medicine (2021)