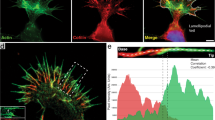
Abstract
Filopodial actin bundles guide microtubule assembly in the growth cone peripheral (P) domain and retrograde actin-network flow simultaneously transports microtubules rearward. Therefore, microtubule-end position is determined by the sum of microtubule assembly and retrograde transport rates. However, how filopodia actually affect microtubule assembly dynamics is unknown. To address this issue we quantitatively assessed microtubule and actin dynamics before and after selective removal of filopodia. Filopodium removal had surprisingly little effect on retrograde actin-flow rates or underlying network structures, but resulted in an approximate doubling of peripheral microtubule density and deeper penetration of microtubules into the P domain. The latter stemmed from less efficient coupling of microtubules to remaining actin networks and not from a change in microtubule polymer dynamics. Loss of filopodia also resulted in increased lateral microtubule movements and a more randomized microtubule distribution in the P domain. In summary, filopodia do not seem to be formally required for microtubule advance; however, their presence ensures radial distribution of microtubules in the P domain and facilitates microtubule transport by retrograde flow. The resulting dynamic steady state has interesting implications for rapid microtubule-positioning responses in the P domain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lewis, A.K. & Bridgman, P. C. Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J. Cell Biol. 119, 1219–1243 (1992).
Song, H.J. & Poo, M. M. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 9, 355–363 (1999).
Dent, E.W. & Gertler, F. B. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron 40, 209–227 (2003).
Koleske, A.J. Do filopodia enable the growth cone to find its way? Sci STKE pe20 (2003).
Zhou, F.Q., Waterman-Storer, C. M. & Cohan, C. S. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J. Cell Biol. 157, 839–849 (2002).
Schaefer, A.W., Kabir, N. & Forscher, P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 158, 139–152 (2002).
Zhou, F.Q. & Cohan, C. S. How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 58, 84–91 (2004).
Challacombe, J.F., Snow, D. M. & Letourneau, P. C. Dynamic microtubule ends are required for growth cone turning to avoid an inhibitory guidance cue. J. Neurosci. 17, 3085–3095 (1997).
Zheng, J.Q., Wan, J. J. & Poo, M. M. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J. Neurosci. 16, 1140–1149 (1996).
Buck, K.B. & Zheng, J. Q. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 22, 9358–9367 (2002).
Suter, D.M., Schaefer, A. W. & Forscher, P. Microtubule dynamics are necessary for SRC family kinase-dependent growth cone steering. Curr. Biol. 14, 1194–1199 (2004).
Medeiros, N.A., Burnette, D. T. & Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nature Cell Biol. 8, 216–226 (2006).
Ji, L. & Danuser, G. Tracking quasi-stationary flow of weak fluorescent signals by adaptive multi-frame correlation. J. Microsc. 220, 150–167 (2005).
Hu, K., Ji, L., Applegate, K.T., Danuser, G. & Waterman-Storer, C. M. Differential transmission of actin motion within focal adhesions. Science 315, 111–115 (2007).
Danuser, G. & Waterman-Storer, C.M. Quantitative fluorescent speckle microscopy of cytoskeleton dynamics. Annu. Rev. Biophys. Biomol. Struct. 35, 361–387 (2006).
Zhang, X.F., Schaefer, A. W., Burnette, D. T., Schoonderwoert, V. T. & Forscher, P. Rho-dependent contractile responses in the neuronal growth cone are independent of classical peripheral retrograde actin flow. Neuron 40, 931–944 (2003).
Lin, C.H. & Forscher, P. Cytoskeletal remodeling during growth cone-target interactions. J. Cell Biol. 121, 1369–1383 (1993).
Waterman-Storer, C.M. & Salmon, E. D. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J. Cell Biol. 139, 417–434 (1997).
Marsh, L. & Letourneau, P.C. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J. Cell Biol. 99, 2041–2047 (1984).
Letourneau, P.C., Shattuck, T. A. & Ressler, A. H. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil. Cytoskeleton 8, 193–209 (1987).
Bentley, D. & Toroian-Raymond, A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature 323, 712–715 (1986).
Bear, J.E. et al. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521 (2002).
Svitkina, T.M., Verkhovsky, A. B. & Borisy, G. G. Improved procedures for electron microscopic visualization of the cytoskeleton of cultured cells. J. Struct. Biol. 115, 290–303 (1995).
Lebrand, C. et al. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42, 37–49 (2004).
Salmon, W.C., Adams, M. C. & Waterman-Storer, C. M. Dual-wavelength fluorescent speckle microscopy reveals coupling of microtubule and actin movements in migrating cells. J. Cell Biol. 158, 31–37 (2002).
Ponti, A., Machacek, M., Gupton, S.L., Waterman-Storer, C. M. & Danuser, G. Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 (2004).
Mogilner, A. & Rubinstein, B. The physics of filopodial protrusion. Biophysical J. 89, 782–795 (2005).
Torreano, P.J., Waterman-Storer, C. M. & Cohan, C. S. The effects of collapsing factors on F-actin content and microtubule distribution of Helisoma growth cones. Cell Motil. Cytoskeleton 60, 166–179 (2005).
Forscher, P. & Smith, S.J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516 (1988).
Walker, R.A. et al. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J. Cell Biol. 107, 1437–1448 (1988).
Acknowledgements
The authors would like to thank members of the Forscher lab for helpful comments and discussion. We thank B. Piekos for training D.B. in electron microscopy. We also thank A. Koleske and T. Pollard for critical reading and their insightful comments. This work was supported NIH grants RO1-NS28695 and RO1-NS051786 to P.F., RO1-GM67230 and U54-GM64346 (Cell Migration Consortium) to G.D. and the Nikon Partners-in-Research Program.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary movie legends (PDF 92 kb)
Supplementary Information
Supplementary Movie 1 (MOV 1849 kb)
Supplementary Information
Supplementary Movie 2 (MOV 2035 kb)
Supplementary Information
Supplementary Movie 3 (MOV 1235 kb)
Supplementary Information
Supplementary Movie 4 (MOV 3132 kb)
Supplementary Information
Supplementary Movie 5 (MOV 4130 kb)
Supplementary Information
Supplementary Movie 6 (MOV 2854 kb)
Rights and permissions
About this article
Cite this article
Burnette, D., Schaefer, A., Ji, L. et al. Filopodial actin bundles are not necessary for microtubule advance into the peripheral domain of Aplysia neuronal growth cones. Nat Cell Biol 9, 1360–1369 (2007). https://doi.org/10.1038/ncb1655
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1655
This article is cited by
-
Quantitative high-precision imaging of myosin-dependent filamentous actin dynamics
Journal of Muscle Research and Cell Motility (2020)
-
Actin–microtubule crosstalk in cell biology
Nature Reviews Molecular Cell Biology (2019)
-
Permeation of Fluorophore-Conjugated Phalloidin into Live Hair Cells of the Inner Ear Is Modulated by P2Y Receptors
Journal of the Association for Research in Otolaryngology (2014)
-
Growth cone-specific functions of XMAP215 in restricting microtubule dynamics and promoting axonal outgrowth
Neural Development (2013)
-
A role for actin arcs in the leading-edge advance of migrating cells
Nature Cell Biology (2011)