Abstract
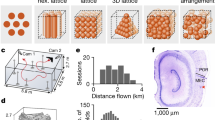
Place-modulated activity among neurons in the hippocampal formation presents a means to organize contextual information in the service of memory formation and recall1,2. One particular spatial representation, that of grid cells, has been observed in the entorhinal cortex (EC) of rats and bats3,4,5, but has yet to be described in single units in primates. Here we examined spatial representations in the EC of head-fixed monkeys performing a free-viewing visual memory task6,7. Individual neurons were identified in the primate EC that emitted action potentials when the monkey fixated multiple discrete locations in the visual field in each of many sequentially presented complex images. These firing fields possessed spatial periodicity similar to a triangular tiling with a corresponding well-defined hexagonal structure in the spatial autocorrelation. Further, these neurons showed theta-band oscillatory activity and changing spatial scale as a function of distance from the rhinal sulcus, which is consistent with previous findings in rodents4,8,9,10. These spatial representations may provide a framework to anchor the encoding of stimulus content in a complex visual scene. Together, our results provide a direct demonstration of grid cells in the primate and suggest that EC neurons encode space during visual exploration, even without locomotion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
28 November 2012
Figs 1–3 were replaced with higher resolution versions.
References
Ekstrom, A. D. et al. Cellular networks underlying human spatial navigation. Nature 425, 184–188 (2003)
Moser, E. I., Kropff, E. & Moser, M.-B. Place cells, grid cells, and the brain’s spatial representation system. Annu. Rev. Neurosci. 31, 69–89 (2008)
Yartsev, M. M., Witter, M. P. & Ulanovsky, N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature 479, 103–107 (2011)
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E. I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005)
Solstad, T., Boccara, C. N., Kropff, E., Moser, M.-B. & Moser, E. I. Representation of geometric borders in the entorhinal cortex. Science 322, 1865–1868 (2008)
Jutras, M. J. & Buffalo, E. A. Recognition memory signals in the macaque hippocampus. Proc. Natl Acad. Sci. USA 107, 401–406 (2010)
Jutras, M. J., Fries, P. & Buffalo, E. A. Gamma-band synchronization in the macaque hippocampus and memory formation. J. Neurosci. 29, 12521–12531 (2009)
Hafting, T., Fyhn, M., Bonnevie, T., Moser, M.-B. & Moser, E. I. Hippocampus-independent phase precession in entorhinal grid cells. Nature 453, 1248–1252 (2008)
Koenig, J., Linder, A. N., Leutgeb, J. K. & Leutgeb, S. The spatial periodicity of grid cells is not sustained during reduced theta oscillations. Science 332, 592–595 (2011)
Brandon, M. P. et al. Reduction of theta rhythm dissociates grid cell spatial periodicity from directional tuning. Science 332, 595–599 (2011)
Rolls, E. T. Spatial view cells and the representation of place in the primate hippocampus. Hippocampus 9, 467–480 (1999)
Tamura, R., Ono, T., Fukuda, M. & Nakamura, K. Spatial responsiveness of monkey hippocampal neurons to various visual and auditory stimuli. Hippocampus 2, 307–322 (1992)
Ono, T., Nakamura, K., Nishijo, H. & Eifuku, S. Monkey hippocampal neurons related to spatial and nonspatial functions. J. Neurophysiol. 70, 1516–1529 (1993)
Robertson, R. G., Rolls, E. T., Georges-François, P. & Panzeri, S. Head direction cells in the primate pre-subiculum. Hippocampus 9, 206–219 (1999)
Suzuki, W. A., Miller, E. K. & Desimone, R. Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 78, 1062–1081 (1997)
Doeller, C. F., Barry, C. & Burgess, N. Evidence for grid cells in a human memory network. Nature 463, 657–661 (2010)
Witter, M. P., Wouterlood, F. G., Naber, P. A. & Van Haeften, T. Anatomical organization of the parahippocampal-hippocampal network. Ann. NY Acad. Sci. 911, 1–24 (2000)
Insausti, R. & Amaral, D. G. Entorhinal cortex of the monkey: IV. Topographical and laminar organization of cortical afferents. J. Comp. Neurol. 509, 608–641 (2008)
Witter, M. P. & Amaral, D. G. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. J. Comp. Neurol. 307, 437–459 (1991)
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006)
Kjelstrup, K. B. et al. Finite scale of spatial representation in the hippocampus. Science 321, 140–143 (2008)
Canto, C. B., Wouterlood, F. G. & Witter, M. P. What does the anatomical organization of the entorhinal cortex tell us? Neural Plast. 2008, 381243 (2008)
Burgess, N., Barry, C. & Keefe, J. O. An oscillatory interference model of grid cell firing. Hippocampus 17, 801–812 (2007)
Stewart, M. & Fox, S. E. Hippocampal theta activity in monkeys. Brain Res. 538, 59–63 (1991)
Ekstrom, A. D. et al. Human hippocampal theta activity during virtual navigation. Hippocampus 15, 881–889 (2005)
Mizuseki, K., Sirota, A., Pastalkova, E. & Buzsáki, G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280 (2009)
Langston, R. F. et al. Development of the spatial representation system in the rat. Science 328, 1576–1580 (2010)
Burgess, N. & O’Keefe, J. Models of place and grid cell firing and theta rhythmicity. Curr. Opin. Neurobiol. 21, 734–744 (2011)
McNaughton, B. L., Battaglia, F. P., Jensen, O., Moser, E. I. & Moser, M.-B. Path integration and the neural basis of the “cognitive map”. Nature Rev. Neurosci. 7, 663–678 (2006)
Sommer, M. A. & Wurtz, R. H. A pathway in primate brain for internal monitoring of movements. Science 296, 1480–1482 (2002)
Acknowledgements
We thank S. Potter, C. Erickson, J. Manns, M. Meister and K. Dunne for comments on the manuscript, and M. Tompkins and D. Solyst for assistance with experiments. This project was funded by the National Institute of Mental Health, R01MH093807 (E.A.B.), R01MH080007 (E.A.B.), MH082559 (M.J.J.), the National Center for Research Resources P51RR165, and is currently supported by the Office of Research Infrastructure Programs/OD P51OD11132. N.J.K. was supported by the NSF IGERT program (DGE-0333411).
Author information
Authors and Affiliations
Contributions
E.A.B. and N.J.K. designed the research, N.J.K. collected the data from the entorhinal cortex, M.J.J. collected data from the hippocampus, N.J.K. performed the analyses, and N.J.K. and E.A.B. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9, Supplementary Methods, Supplementary Table 1 and additional references. (PDF 1200 kb)
Rights and permissions
About this article
Cite this article
Killian, N., Jutras, M. & Buffalo, E. A map of visual space in the primate entorhinal cortex. Nature 491, 761–764 (2012). https://doi.org/10.1038/nature11587
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11587
This article is cited by
-
A spatial transformation-based CAN model for information integration within grid cell modules
Cognitive Neurodynamics (2024)
-
Entorhinal grid-like codes and time-locked network dynamics track others navigating through space
Nature Communications (2023)
-
Neural Correlates of Spatial Navigation in Primate Hippocampus
Neuroscience Bulletin (2023)
-
Nucleus incertus projections to rat medial septum and entorhinal cortex: rare collateralization and septal-gating of temporal lobe theta rhythm activity
Brain Structure and Function (2023)
-
Perception of Color and Its Encoding in the Cortex in Primates
Neuroscience and Behavioral Physiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.