Abstract
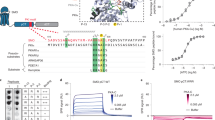
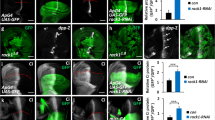
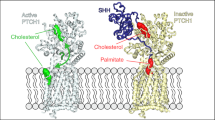
The hedgehog (Hh) signalling pathway has an evolutionarily conserved role in patterning fields of cells during metazoan development, and is inappropriately activated in cancer1,2. Hh pathway activity is absolutely dependent on signalling by the seven-transmembrane protein smoothened (Smo), which is regulated by the Hh receptor patched (Ptc). Smo signals to an intracellular multi-protein complex containing the Kinesin related protein Costal2 (Cos2), the protein kinase Fused (Fu) and the transcription factor Cubitus interruptus (Ci)3. In the absence of Hh, this complex regulates the cleavage of full-length Ci to a truncated repressor protein, Ci75, in a process that is dependent on the proteasome and priming phosphorylations by Protein kinase A (PKA)4. Binding of Hh to Ptc blocks Ptc-mediated Smo inhibition, allowing Smo to signal to the intracellular components to attenuate Ci cleavage. Because of its homology with the Frizzled family of G-protein-coupled receptors (GPCR)5, a likely candidate for an immediate Smo effector would be a heterotrimeric G protein. However, the role that G proteins may have in Hh signal transduction is unclear and quite controversial6,7,8,9,10, which has led to widespread speculation that Smo signals through a variety of novel G-protein-independent mechanisms. Here we present in vitro and in vivo evidence in Drosophila that Smo activates a G protein to modulate intracellular cyclic AMP levels in response to Hh. Our results demonstrate that Smo functions as a canonical GPCR, which signals through Gαi to regulate Hh pathway activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
18 December 2008
The AOP version of this paper contained an error in the Figure 4 legend. This was corrected on 18 December 2008.
References
Mullor, J. L., Sanchez, P. & Altaba, A. R. Pathways and consequences: Hedgehog signaling in human disease. Trends Cell Biol. 12, 562–569 (2002)
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001)
Robbins, D. J. et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90, 225–234 (1997)
Price, M. A. & Kalderon, D. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell 108, 823–835 (2002)
Alcedo, J., Ayzenzon, M., Von Ohlen, T., Noll, M. & Hooper, J. E. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86, 221–232 (1996)
DeCamp, D. L., Thompson, T. M., de Sauvage, F. J. & Lerner, M. R. Smoothened activates Gαi-mediated signaling in frog melanophores. J. Biol. Chem. 275, 26322–26327 (2000)
Low, W. C. et al. The decoupling of Smoothened from Gαi proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Dev. Biol. 321, 188–196 (2008)
Kasai, K. et al. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells 9, 49–58 (2004)
Riobo, N. A., Saucy, B., Dilizio, C. & Manning, D. R. Activation of heterotrimeric G proteins by Smoothened. Proc. Natl Acad. Sci. USA 103, 12607–12612 (2006)
Murone, M., Rosenthal, A. & de Sauvage, F. J. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr. Biol. 9, 76–84 (1999)
Chen, C. H. et al. Nuclear trafficking of Cubitus interruptus in the transcriptional regulation of Hedgehog target gene expression. Cell 98, 305–316 (1999)
Schaefer, M., Petronczki, M., Dorner, D., Forte, M. & Knoblich, J. A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell 107, 183–194 (2001)
Muller, B. & Basler, K. The repressor and activator forms of Cubitus interruptus control Hedgehog target genes through common generic gli-binding sites. Development 127, 2999–3007 (2000)
Methot, N. & Basler, K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell 96, 819–831 (1999)
Capdevila, J. & Guerrero, I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13, 4459–4468 (1994)
Collins, R. T. & Cohen, S. M. A genetic screen in Drosophila for identifying novel components of the hedgehog signaling pathway. Genetics 170, 173–184 (2005)
Yu, F., Cai, Y., Kaushik, R., Yang, X. & Chia, W. Distinct roles of Gαi and Gβ13F subunits of the heterotrimeric G protein complex in the mediation of Drosophila neuroblast asymmetric divisions. J. Cell Biol. 162, 623–633 (2003)
Hampoelz, B., Hoeller, O., Bowman, S. K., Dunican, D. & Knoblich, J. A. Drosophila Ric-8 is essential for plasma-membrane localization of heterotrimeric G proteins. Nature Cell Biol. 7, 1099–1105 (2005)
Affolter, M. & Basler, K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nature Rev. Genet. 8, 663–674 (2007)
Aegerter-Wilmsen, T., Aegerter, C. M., Hafen, E. & Basler, K. Model for the regulation of size in the wing imaginal disc of Drosophila . Mech. Dev. 124, 318–326 (2007)
Martin-Blanco, E., Pastor-Pareja, J. C. & Garcia-Bellido, A. JNK and decapentaplegic signaling control adhesiveness and cytoskeleton dynamics during thorax closure in Drosophila . Proc. Natl Acad. Sci. USA 97, 7888–7893 (2000)
Pena-Rangel, M. T., Rodriguez, I. & Riesgo-Escovar, J. R. A misexpression study examining dorsal thorax formation in Drosophila melanogaster . Genetics 160, 1035–1050 (2002)
Ohlmeyer, J. T. & Kalderon, D. Dual pathways for induction of wingless expression by protein kinase A and Hedgehog in Drosophila embryos. Genes Dev. 11, 2250–2258 (1997)
Apionishev, S., Katanayeva, N. M., Marks, S. A., Kalderon, D. & Tomlinson, A. Drosophila Smoothened phosphorylation sites essential for Hedgehog signal transduction. Nature Cell Biol. 7, 86–92 (2005)
Davis, R. L. & Kiger, J. A. Dunce mutants of Drosophila melanogaster: mutants defective in the cyclic AMP phosphodiesterase enzyme system. J. Cell Biol. 90, 101–107 (1981)
Ogden, S. K., Ascano, M., Stegman, M. A. & Robbins, D. J. Regulation of Hedgehog signaling: a complex story. Biochem. Pharmacol. 67, 805–814 (2004)
Jia, J. & Jiang, J. Decoding the Hedgehog signal in animal development. Cell. Mol. Life Sci. 63, 1249–1265 (2006)
Stegman, M. A. et al. The Kinesin-related protein Costal2 associates with membranes in a Hedgehog-sensitive, Smoothened-independent manner. J. Biol. Chem. 279, 7064–7071 (2004)
Ogden, S. K. et al. Smoothened regulates activator and repressor functions of Hedgehog signaling via two distinct mechanisms. J. Biol. Chem. 281, 7237–7243 (2006)
Stitham, J., Stojanovic, A., Ross, L. A., Blount, A. C. & Hwa, J. Clusters of transmembrane residues are critical for human prostacyclin receptor activation. Biochemistry 43, 8974–8986 (2004)
Motzny, C. K. & Holmgren, R. The Drosophila cubitus interruptus protein and its role in the wingless and hedgehog signal transduction pathways. Mech. Dev. 52, 137–150 (1995)
Stegman, M. A. et al. Identification of a tetrameric hedgehog signaling complex. J. Biol. Chem. 275, 21809–21812 (2000)
Ascano, M., Nybakken, K. E., Sosinski, J., Stegman, M. A. & Robbins, D. J. The carboxyl-terminal domain of the protein kinase fused can function as a dominant inhibitor of hedgehog signaling. Mol. Cell. Biol. 22, 1555–1566 (2002)
Brand, A. H. & Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993)
Patel, N. H., Kornberg, T. B. & Goodman, C. S. Expression of engrailed during segmentation in grasshopper and crayfish. Development 107, 201–212 (1989)
Lum, L. et al. Hedgehog signal transduction via Smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol. Cell 12, 1261–1274 (2003)
Ogden, S. K. et al. Identification of a functional interaction between the transmembrane protein Smoothened and the kinesin-related protein Costal2. Curr. Biol. 13, 1998–2003 (2003)
Acknowledgements
This work was supported by National Institutes of Health grants CA82628 (D.J.R.) and HL074190 (J.H.). We are grateful to J. Knoblich for the Gαi antibody and Gαi flies; X. Yang and W. Chia for Gαi flies; D. Casso and T. Kornberg for Ptc antibody and UAS-Smo5A flies; J. Hooper for UAS-Gαi and UAS-GαiQ205L flies; K. Basler, D. Kalderon and P. Ingham for smo alleles; and L. Lum for Smo antibody. All additional fly stocks were provided by the Bloomington Stock Center. Gαi and Gαo cDNAs were obtained from the Drosophila Genomics Resource Center, Bloomington. E. Lee provided Gαs cDNA. We thank the Dartmouth microscopy core for their assistance. We would also like to thank K. Black for technical assistance and members of the Robbins laboratory, E. Lee, J. Hutchinson, R. Taussig, R. Ostrom and C. Pikielny for thoughtful discussion during the course of this work.
Author Contributions S.K.O. was involved in the design, execution and analysis of experiments, and in writing the manuscript. D.L.F. assisted in design and execution of dsRNA experiments and sequencing of Gαi alleles. J.H. was involved in design and execution of cAMP assays. N.S.S. assisted in execution of cAMP assays. Y.F.A. consulted on design and interpretation of genetic experiments. D.J.R. was involved in design and analysis of experiments and writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S4 with Legends. (PDF 1030 kb)
Rights and permissions
About this article
Cite this article
Ogden, S., Fei, D., Schilling, N. et al. G protein Gαi functions immediately downstream of Smoothened in Hedgehog signalling. Nature 456, 967–970 (2008). https://doi.org/10.1038/nature07459
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07459
This article is cited by
-
GPR161 structure uncovers the redundant role of sterol-regulated ciliary cAMP signaling in the Hedgehog pathway
Nature Structural & Molecular Biology (2024)
-
Hedgehog signaling in tissue homeostasis, cancers, and targeted therapies
Signal Transduction and Targeted Therapy (2023)
-
Cholesterylation of Smoothened is a calcium-accelerated autoreaction involving an intramolecular ester intermediate
Cell Research (2022)
-
Novel de novo pathogenic variant in the GNAI1 gene as a cause of severe disorders of intellectual development
Journal of Human Genetics (2022)
-
Control of the Hedgehog pathway by compartmentalized PKA in the primary cilium
Science China Life Sciences (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.