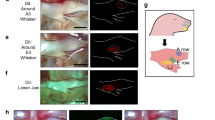
Abstract
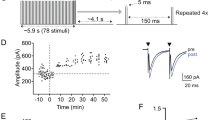
Spontaneous activity in the developing auditory system is required for neuronal survival as well as the refinement and maintenance of tonotopic maps in the brain. However, the mechanisms responsible for initiating auditory nerve firing in the absence of sound have not been determined. Here we show that supporting cells in the developing rat cochlea spontaneously release ATP, which causes nearby inner hair cells to depolarize and release glutamate, triggering discrete bursts of action potentials in primary auditory neurons. This endogenous, ATP-mediated signalling synchronizes the output of neighbouring inner hair cells, which may help refine tonotopic maps in the brain. Spontaneous ATP-dependent signalling rapidly subsides after the onset of hearing, thereby preventing this experience-independent activity from interfering with accurate encoding of sound. These data indicate that supporting cells in the organ of Corti initiate electrical activity in auditory nerves before hearing, pointing to an essential role for peripheral, non-sensory cells in the development of central auditory pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Geal-Dor, M., Freeman, S., Li, G. & Sohmer, H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear. Res. 69, 236–242 (1993)
Puel, J. L. & Uziel, A. Correlative development of cochlear action potential sensitivity, latency, and frequency selectivity. Brain Res. 465, 179–188 (1987)
Glowatzki, E. & Fuchs, P. A. Transmitter release at the hair cell ribbon synapse. Nature Neurosci. 5, 147–154 (2002)
Gummer, A. W. & Mark, R. F. Patterned neural activity in brain stem auditory areas of a prehearing mammal, the tammar wallaby (Macropus eugenii). Neuroreport 5, 685–688 (1994)
Jones, T. A., Jones, S. M. & Paggett, K. C. Primordial rhythmic bursting in embryonic cochlear ganglion cells. J. Neurosci. 21, 8129–8135 (2001)
Jones, T. A., Leake, P. A., Snyder, R. L., Stakhovskaya, O. & Bonham, B. H. Spontaneous discharge patterns in cochlear spiral ganglion cells prior to the onset of hearing in cats. J. Neurophysiol. doi: 10.1152/jn.00472.2007 (8 August 2007)
Walsh, E. J. & McGee, J. Postnatal development of auditory nerve and cochlear nucleus neuronal responses in kittens. Hear. Res. 28, 97–116 (1987)
Lippe, W. R. Rhythmic spontaneous activity in the developing avian auditory system. J. Neurosci. 14, 1486–1495 (1994)
Retzius, G. Das Gehörorgan der Wirbelthiere. II. Das Gehörorgan der Reptilien, der Vögel und Säugethiere (Samson & Wallin, Stockholm, 1884)
Hinojosa, R. A note on development of Corti’s organ. Acta Otolaryngol. (Stockh.) 84, 238–251 (1977)
Wada, T. Anatomical and physiological studies on the growth of the inner ear of the albino rat. Am. Anat. Mem. 10, 1–174 (1923)
Eybalin, M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol. Rev. 73, 309–373 (1993)
Glowatzki, E. & Fuchs, P. A. Cholinergic synaptic inhibition of inner hair cells in the neonatal mammalian cochlea. Science 288, 2366–2368 (2000)
Fuchs, P. A., Evans, M. G. & Murrow, B. W. Calcium currents in hair cells isolated from the cochlea of the chick. J. Physiol. (Lond.) 429, 553–568 (1990)
Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007)
Housley, G. D. Physiological effects of extracellular nucleotides in the inner ear. Clin. Exp. Pharmacol. Physiol. 27, 575–580 (2000)
North, R. A. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 (2002)
Bennett, M. V., Contreras, J. E., Bukauskas, F. F. & Saez, J. C. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci. 26, 610–617 (2003)
Forge, A. et al. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessment of connexin composition in mammals. J. Comp. Neurol. 467, 207–231 (2003)
Zhao, H. B. Connexin26 is responsible for anionic molecule permeability in the cochlea for intercellular signalling and metabolic communications. Eur. J. Neurosci. 21, 1859–1868 (2005)
Charles, A. & Giaume, C. Intercellular Calcium Waves in Astrocytes: Underlying Mechanisms and Functional Significance (eds Volterra, A., Magistretti, P. J. & Haydon, P. G.) (Oxford Univ. Press, Oxford, 2002)
Piazza, V., Ciubotaru, C. D., Gale, J. E. & Mammano, F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium 41, 77–86 (2007)
Salih, S. G., Jagger, D. J. & Housley, G. D. ATP-gated currents in rat primary auditory neurones in situ arise from a heteromultimetric P2X receptor subunit assembly. Neuropharmacology 42, 386–395 (2002)
Sugasawa, M., Erostegui, C., Blanchet, C. & Dulon, D. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J. Physiol. (Lond.) 491, 707–718 (1996)
Jones, T. A. & Jones, S. M. Spontaneous activity in the statoacoustic ganglion of the chicken embryo. J. Neurophysiol. 83, 1452–1468 (2000)
Rübsamen, R. & Lippe, W. R. in Development of the Auditory System (eds Rubel, E. W., Popper, A. N. & Fay, R. R.) 193–270 (Springer, New York, 1998)
Müller, M. Frequency representation in the rat cochlea. Hear. Res. 51, 247–254 (1991)
Beutner, D. & Moser, T. The presynaptic function of mouse cochlear inner hair cells during development of hearing. J. Neurosci. 21, 4593–4599 (2001)
Marcotti, W., Johnson, S. L., Rüsch, A. & Kros, C. J. Sodium and calcium currents shape action potentials in immature mouse inner hair cells. J. Physiol. (Lond.) 552, 743–761 (2003)
Friauf, E. & Lohmann, C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 297, 187–195 (1999)
Kandler, K. Activity-dependent organization of inhibitory circuits: lessons from the auditory system. Curr. Opin. Neurobiol. 14, 96–104 (2004)
Rubel, E. W. & Fritzsch, B. Auditory system development: primary auditory neurons and their targets. Annu. Rev. Neurosci. 25, 51–101 (2002)
Leao, R. N. et al. Topographic organization in the auditory brainstem of juvenile mice is disrupted in congenital deafness. J. Physiol. (Lond.) 571, 563–578 (2006)
Leake, P. A., Hradek, G. T., Chair, L. & Snyder, R. L. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J. Comp. Neurol. 497, 13–31 (2006)
Gabriele, M. L., Brunso-Bechtold, J. K. & Henkel, C. K. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J. Neurosci. 20, 6939–6949 (2000)
Kros, C. J., Rüppersberg, J. P. & Rüsch, A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature 394, 281–284 (1998)
Marcotti, W., Johnson, S. L., Holley, M. C. & Kros, C. J. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J. Physiol. (Lond.) 548, 383–400 (2003)
Katz, L. C. & Shatz, C. J. Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138 (1996)
Kotak, V. C. & Sanes, D. H. Synaptically evoked prolonged depolarizations in the developing auditory system. J. Neurophysiol. 74, 1611–1620 (1995)
Huberman, A. D. Mechanisms of eye-specific visual circuit development. Curr. Opin. Neurobiol. 17, 73–80 (2007)
Erazo-Fischer, E., Striessnig, J. & Taschenberger, H. The role of physiological afferent nerve activity during in vivo maturation of the calyx of Held synapse. J. Neurosci. 27, 1725–1737 (2007)
Lagostena, L. & Mammano, F. Intracellular calcium dynamics and membrane conductance changes evoked by Deiters’ cell purinoceptor activation in the organ of Corti. Cell Calcium 29, 191–198 (2001)
Gale, J. E., Piazza, V., Ciubotaru, C. D. & Mammano, F. A mechanism for sensing noise damage in the inner ear. Curr. Biol. 14, 526–529 (2004)
Fields, R. D. & Burnstock, G. Purinergic signalling in neuron–glia interactions. Nature Rev. Neurosci. 7, 423–436 (2006)
Nedergaard, M., Ransom, B. & Goldman, S. A. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26, 523–530 (2003)
Glowatzki, E. et al. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J. Neurosci. 26, 7659–7664 (2006)
Acknowledgements
We thank J.-H. Kong for help with preliminary experiments, and P. Fuchs, J. Howell and M. Lahne for discussions. This work was supported by a Royal Society University Research Fellowship (to J.E.G.), National Institutes of Health Grants (to E.G. and D.E.B.) and the Deafness Research Foundation (to D.E.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-6 with Legends and Supplementary Video Legends. Supplementary Figures 1-6 and their legends provide evidence for correlation between spontaneous field potentials and inward currents in supporting cells, additional pharmacology of spontaneous extracellular potentials and spontaneous inward currents in supporting cells and inner hair cells, and further characterization of spontaneous optical changes and ATP-elicited Ca2+ transients within the developing cochlea. (PDF 12570 kb)
Supplementary Video 1a
The file contains Supplementary Video 1a showing time lapse of the unprocessed transmitted light (IR-DIC) signal acquired from a P8 rat organ of Corti illustrates the intrinsic optical changes that spontaneously occur in the supporting cells of Kölliker’s organ. (MOV 6687 kb)
Supplementary Video 1b
The file contains Supplementary Video 1b showing time lapse of Supplementary Video 1 after image subtraction, providing an index of transmitted light change over time. (MOV 10102 kb)
Supplementary Video 1c
The file contains Supplementary Video 1c showing time lapse of Supplementary Video 1 after image subtraction, thresholding and pseudocoloring. (MOV 2499 kb)
Rights and permissions
About this article
Cite this article
Tritsch, N., Yi, E., Gale, J. et al. The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007). https://doi.org/10.1038/nature06233
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06233
This article is cited by
-
Effects of Noise Damage on the Purinergic Signal of Cochlear Spiral Ganglion Cells in Guinea Pigs
Molecular Biotechnology (2024)
-
TRPA1 activation in non-sensory supporting cells contributes to regulation of cochlear sensitivity after acoustic trauma
Nature Communications (2023)
-
Synchronized activity of sensory neurons initiates cortical synchrony in a model of neuropathic pain
Nature Communications (2023)
-
Early radial positional information in the cochlea is optimized by a precise linear BMP gradient and enhanced by SOX2
Scientific Reports (2023)
-
Step by step: cells with multiple functions in cortical circuit assembly
Nature Reviews Neuroscience (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.