Abstract
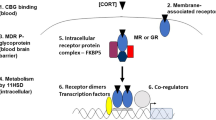
Maladaptive glucocorticoid effects contribute to stress-related psychopathology. The glucocorticoid receptor (GR) that mediates many of these effects uses multiple signaling pathways. We have tested the hypothesis that manipulation of downstream factors (‘coregulators’) can abrogate potentially maladaptive GR-mediated effects on fear-motivated behavior that are linked to corticotropin releasing hormone (CRH). For this purpose the expression ratio of two splice variants of steroid receptor coactivator-1 (SRC-1) was altered via antisense-mediated ‘exon-skipping’ in the central amygdala of the mouse brain. We observed that a change in splicing towards the repressive isoform SRC-1a strongly reduced glucocorticoid-induced responsiveness of Crh mRNA expression and increased methylation of the Crh promoter. The transcriptional GR target gene Fkbp5 remained responsive to glucocorticoids, indicating gene specificity of the effect. The shift of the SRC-1 splice variants altered glucocorticoid-dependent exploratory behavior and attenuated consolidation of contextual fear memory. In conclusion, our findings demonstrate that manipulation of GR signaling pathways related to the Crh gene can selectively diminish potentially maladaptive effects of glucocorticoids.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
de Kloet ER, Fitzsimons CP, Datson NA, Meijer OC, Vreugdenhil E . Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res 2009; 1293: 129–141.
de Kloet ER, Joels M, Holsboer F . Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475.
Dasgupta S, Lonard DM, O'Malley BW . Nuclear receptor coactivators: master regulators of human health and disease. Annu Rev Med 2014; 65: 279–292.
Tetel MJ, Auger AP, Charlier TD . Who's in charge? Nuclear receptor coactivator and corepressor function in brain and behavior. Front Neuroendocrinol 2009; 30: 328–342.
Zalachoras I, Houtman R, Meijer OC . Understanding stress-effects in the brain via transcriptional signal transduction pathways. Neuroscience 2013; 242: 97–109.
Flandreau EI, Bourke CH, Ressler KJ, Vale WW, Nemeroff CB, Owens MJ . Escitalopram alters gene expression and HPA axis reactivity in rats following chronic overexpression of corticotropin-releasing factor from the central amygdala. Psychoneuroendocrinology 2013; 38: 1349–1361.
Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB . Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 2012; 37: 27–38.
Kolber BJ, Roberts MS, Howell MP, Wozniak DF, Sands MS, Muglia LJ . Central amygdala glucocorticoid receptor action promotes fear-associated CRH activation and conditioning. Proc Natl Acad Sci USA 2008; 105: 12004–12009.
Stenzel-Poore M, Heinrichs S, Rivest S, Koob G, Vale W . Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci 1994; 14: 2579–2584.
Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry 2009; 14: 37–50.
Kovács KJ . CRH: the link between hormonal-, metabolic- and behavioral responses to stress. J Chem Neuroanat 2013; 54: 25–33.
Makino S, Gold PW, Schulkin J . Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res 1994; 640: 105–112.
Tognoni CM, Chadwick JJG, Ackeifi CA, Tetel MJ . Nuclear receptor coactivators are coexpressed with steroid receptors and regulated by estradiol in mouse brain. Neuroendocrinology 2011; 94: 49–57.
Meijer OC, Kalkhoven E, van der Laan S, Steenbergen PJ, Houtman SH, Dijkmans TF et al. Steroid receptor coactivator-1 splice variants differentially affect corticosteroid receptor signaling. Endocrinology 2005; 146: 1438–1448.
Lachize S, Apostolakis EM, van der Laan S, Tijssen AM, Xu J, de Kloet ER et al. Steroid receptor coactivator-1 is necessary for regulation of corticotropin-releasing hormone by chronic stress and glucocorticoids. Proc Natl Acad Sci USA 2009; 106: 8038–8042.
van der Laan S, Lachize SB, Vreugdenhil E, de Kloet ER, Meijer OC . Nuclear receptor coregulators differentially modulate induction and glucocorticoid receptor-mediated repression of the corticotropin-releasing hormone gene. Endocrinology 2008; 149: 725–732.
Meijer OC, van der Laan S, Lachize S, Steenbergen PJ, de Kloet ER . Steroid receptor coregulator diversity: what can it mean for the stressed brain? Neuroscience 2006; 138: 891–899.
Meijer OC, Steenbergen PJ, de Kloet ER . Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinology 2000; 141: 2192–2199.
Arechavala-Gomeza V, Khoo B, Aartsma-Rus A . Splicing modulation therapy in the treatment of genetic diseases. Appl Clin Genet 2014; 7: 245–252.
Kalkhoven E, Valentine JE, Heery DM, Parker MG . Isoforms of steroid receptor co-activator 1 differ in their ability to potentiate transcription by the oestrogen receptor. EMBO J 1998; 17: 232–243.
Zalachoras I, Grootaers G, van Weert LT, Aubert Y, de Kreij SR, Datson NA et al. Antisense-mediated isoform switching of steroid receptor coactivator-1 in the central nucleus of the amygdala of the mouse brain. BMC Neurosci 2013; 14: 5.
Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A . Resilience to social stress coincides with functional DNA methylation of the Crf gene in adult mice. Nat Neurosci 2010; 13: 1351–1353.
Sharma D, Bhave S, Gregg E, Uht R . Dexamethasone induces a putative repressor complex and chromatin modifications in the CRH promoter. Mol Endocrinol 2013; 27: 1142–1152.
ter Horst JP, Carobrez AP, van der Mark MH, de Kloet ER, Oitzl MS . Sex differences in fear memory and extinction of mice with forebrain-specific disruption of the mineralocorticoid receptor. Eur J Neurosci 2012; 36: 3096–3102.
Brinks V, Berger S, Gass P, de Kloet ER, Oitzl MS . Mineralocorticoid receptors in control of emotional arousal and fear memory. Horm Behav 2009; 56: 232–238.
Datson NA, Meijer L, Steenbergen PJ, Morsink MC, van der Laan S, Meijer OC et al. Expression profiling in laser-microdissected hippocampal subregions in rat brain reveals large subregion-specific differences in expression. Eur J Neurosci 2004; 20: 2541–2554.
Datson NA, Morsink MC, Steenbergen PJ, Aubert Y, Schlumbohm C, Fuchs E et al. A molecular blueprint of gene expression in hippocampal subregions CA1, CA3, and DG is conserved in the brain of the common marmoset. Hippocampus 2009; 19: 739–752.
Pfaffl MW . A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29: e45.
Parrish RR, Day JJ, Lubin FD . Direct bisulfite sequencing for examination of dna methylation with gene and nucleotide resolution from brain tissues. Curr Protoc Neurosci 2012; 60: 7.24.1–7.24.12.
Hua Y, Sahashi K, Hung G, Rigo F, Passini MA, Bennett CF et al. Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev 2010; 24: 1634–1644.
Heemskerk H, de Winter C, van Kuik P, Heuvelmans N, Sabatelli P, Rimessi P et al. Preclinical PK and PD studies on 2'-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol Ther 2010; 18: 1210–1217.
Evers MM, Tran HD, Zalachoras I, Meijer OC, den Dunnen JT, van Ommen GJ et al. Preventing formation of toxic N-terminal huntingtin fragments through antisense oligonucleotide-mediated protein modification. Nucleic Acid Ther 2014; 24: 4–12.
Evers MM, Tran HD, Zalachoras I, Pepers BA, Meijer OC, den Dunnen JT et al. Ataxin-3 protein modification as a treatment strategy for spinocerebellar ataxia type 3: removal of the CAG containing exon. Neurobiol Dis 2013; 58: 49–56.
Goemans N, Tulinius M, van den Akker J, Burm B, Ekhart P, Heuvelmans N et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med 2011; 364: 1513–1522.
Xu J, Lu Z, Xu M, Pan L, Deng Y, Xie X et al. A heroin addiction severity-associated intronic single nucleotide polymorphism modulates alternative pre-mRNA Splicing of the μ opioid receptor gene OPRM1 via hnRNPH interactions. J Neurosci 2014; 34: 11048–11066.
Fagnani M, Barash Y, Ip JY, Misquitta C, Pan Q, Saltzman AL et al. Functional coordination of alternative splicing in the mammalian central nervous system. Genome Biol 2007; 8: R108.
Ding XF, Anderson CM, Ma H, Hong H, Uht RM, Kushner PJ et al. Nuclear receptor-binding sites of coactivators glucocorticoid receptor interacting protein 1 (GRIP1) and steroid receptor coactivator 1 (SRC-1): multiple motifs with different binding specificities. Mol Endocrinol 1998; 12: 302–313.
van der Doelen RHA, Arnoldussen IA, Ghareh H, van Och L, Homberg JR, Kozicz T . Early life adversity and serotonin transporter gene variation interact to affect DNA methylation of the corticotropin-releasing factor gene promoter region in the adult rat brain. Dev Psychopathol 2015; 27 (Special Issue 01): 123–135.
Refojo D, Schweizer M, Kuehne C, Ehrenberg S, Thoeringer C, Vogl AM et al. Glutamatergic and dopaminergic neurons mediate anxiogenic and anxiolytic effects of CRHR1. Science 2011; 333: 1903–1907.
Pitts MW, Todorovic C, Blank T, Takahashi LK . The central nucleus of the amygdala and corticotropin-releasing factor: insights into contextual fear memory. J Neurosci 2009; 29: 7379–7388.
Pitts MW, Takahashi LK . The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem 2010; 95: 86–91.
Phillips RG, LeDoux JE . Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 1992; 106: 274–285.
Højfeldt JW, Cruz-Rodríguez O, Imaeda Y, Van Dyke AR, Carolan JP, Mapp AK et al. Bifunctional ligands allow deliberate extrinsic reprogramming of the glucocorticoid receptor. Mol Endocrinol 2014; 28: 249–259.
Zalachoras I, Houtman R, Atucha E, Devos R, Tijssen AM, Hu P et al. Differential targeting of brain stress circuits with a selective glucocorticoid receptor modulator. Proc Natl Acad Sci USA 2013; 110: 7910–7915.
Solomon MB, Wulsin AC, Rice T, Wick D, Myers B, McKlveen J et al. The selective glucocorticoid receptor antagonist CORT 108297 decreases neuroendocrine stress responses and immobility in the forced swim test. Horm Behav 2014; 65: 363–371.
Acknowledgements
We thank Ms Hetty Sips for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Rights and permissions
About this article
Cite this article
Zalachoras, I., Verhoeve, S., Toonen, L. et al. Isoform switching of steroid receptor co-activator-1 attenuates glucocorticoid-induced anxiogenic amygdala CRH expression. Mol Psychiatry 21, 1733–1739 (2016). https://doi.org/10.1038/mp.2016.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2016.16
This article is cited by
-
The cortisol switch between vulnerability and resilience
Molecular Psychiatry (2023)
-
Corticosteroid sensitization drives opioid addiction
Molecular Psychiatry (2022)
-
Resetting the Stress System with a Mifepristone Challenge
Cellular and Molecular Neurobiology (2019)
-
Corticosteroid Receptors in the Brain: Transcriptional Mechanisms for Specificity and Context-Dependent Effects
Cellular and Molecular Neurobiology (2019)