Abstract
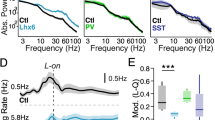
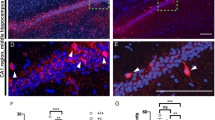
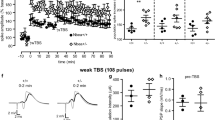
Alterations in glutamatergic transmission onto developing GABAergic systems, in particular onto parvalbumin-positive (Pv+) fast-spiking interneurons, have been proposed as underlying causes of several neurodevelopmental disorders, including schizophrenia and autism. Excitatory glutamatergic transmission, through ionotropic and metabotropic glutamate receptors, is necessary for the correct postnatal development of the Pv+ GABAergic network. We generated mutant mice in which the metabotropic glutamate receptor 5 (mGluR5) was specifically ablated from Pv+ interneurons postnatally, and investigated the consequences of such a manipulation at the cellular, network and systems levels. Deletion of mGluR5 from Pv+ interneurons resulted in reduced numbers of Pv+ neurons and decreased inhibitory currents, as well as alterations in event-related potentials and brain oscillatory activity. These cellular and sensory changes translated into domain-specific memory deficits and increased compulsive-like behaviors, abnormal sensorimotor gating and altered responsiveness to stimulant agents. Our findings suggest a fundamental role for mGluR5 in the development of Pv+ neurons and show that alterations in this system can produce broad-spectrum alterations in brain network activity and behavior that are relevant to neurodevelopmental disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Marín O . Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012; 13: 107–120.
Huang ZJ . Activity-dependent development of inhibitory synapses and innervation pattern: role of GABA signalling and beyond. J Physiology 2009; 587: 1881–1888.
Xue M, Atallah BV, Scanziani M . Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 2014; 511: 596–600.
Bartos M, Vida I, Jonas P . Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci 2007; 8: 45–56.
Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W . Neural synchrony and the development of cortical networks. Trends Cogn Sci 2010; 14: 72–80.
Doischer D, Hosp JA, Yanagawa Y, Obata K, Jonas P, Vida I et al. Postnatal differentiation of basket cells from slow to fast signaling devices. J Neurosci 2008; 28: 12956–12968.
Okaty BW, Miller MN, Sugino K, Hempel CM, Nelson SB . Transcriptional and electrophysiological maturation of neocortical fast-spiking GABAergic interneurons. J Neurosci 2009; 29: 7040–7052.
Sauer JF, Bartos M . Recruitment of early postnatal parvalbumin-positive hippocampal interneurons by GABAergic excitation. J Neurosc 2010; 30: 110–115.
Hensch TK . Critical period regulation. Annu Rev Neurosci 2004; 27: 549–579.
Wang HX, Gao WJ . Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacology 2009; 34: 2028–2040.
Zhang Z, Sun QQ . Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical GABAergic interneurons. Dev Neurobiol 2011; 71: 221–245.
Carlen M, Meletis K, Siegle JH, Cardin JA, Futai K, Vierling-Claassen D et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry 2012; 17: 537–548.
Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci 2010; 13: 76–83.
Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H . NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron 2010; 68: 557–569.
Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron 2007; 53: 591–604.
Niswender CM, Conn PJ . Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 2010; 50: 295–322.
Van Hooft JA, Giuffrida R, Blatow M, Monyer H . Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci 2000; 20: 3544–3551.
Sarihi A, Jiang B, Komaki A, Sohya K, Yanagawa Y, Tsumoto T . Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci 2008; 28: 1224–1235.
Brody SA, Dulawa SC, Conquet F, Geyer MA . Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry 2004; 9: 35–41.
Lu Y-M, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM et al. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci 1997; 17: 5196–5205.
Xu J, Zhu Y, Contractor A, Heinemann SF . mGluR5 has a critical role in inhibitory learning. J Neurosci 2009; 29: 3676–3684.
Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140.
Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, Quick KL et al. IL-6 mediated degeneration of forebrain GABAergic interneurons and cognitive impairment in aged mice through activation of neuronal NADPH oxidase. PLoS One 2009; 4: e5518.
Abercrombie M . Estimation of nuclear population from microtome sections. Anat Rec 1946; 94: 239–247.
Zembrzycki A, Chou SJ, Ashery-Padan R, Stoykova A, O'Leary DD . Sensory cortex limits cortical maps and drives top-down plasticity in thalamocortical circuits. Nat Neurosci 2013; 16: 1060–1067.
Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM . A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci 2006; 26: 1604–1615.
Anderson WW, Collingridge GL . Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J Neurosci Methods 2007; 162: 346–356.
Nadler JJ, Moy SS, Dold G, Simmons N, Perez A, Young NB et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav 2004; 3: 303–314.
Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R . Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009; 204: 361–373.
Semenova S, Geyer MA, Sutcliffe JG, Markou A, Hedlund PB . Inactivation of the 5-HT(7) receptor partially blocks phencyclidine-induced disruption of prepulse inhibition. Biol Psychiatry 2008; 63: 98–105.
Moy SS, Perez A, Koller BH, Duncan GE . Amphetamine-induced disruption of prepulse inhibition in mice with reduced NMDA receptor function. Brain Res 2006; 1089: 186–194.
Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR et al. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol 2005; 3: e159.
Kolber BJ, Montana MC, Carrasquillo Y, Xu J, Heinemann SF, Muglia LJ et al. Activation of metabotropic glutamate receptor 5 in the amygdala modulates pain-like behavior. J Neurosci 2010; 30: 8203–8213.
Ballester-Rosado CJ, Albright MJ, Wu CS, Liao CC, Zhu J, Xu J et al. mGluR5 in cortical excitatory neurons exerts both cell-autonomous and -nonautonomous influences on cortical somatosensory circuit formation. J Neurosci 2010; 30: 16896–16909.
Jew CP, Wu C-S, Sun H, Zhu J, Huang J-Y, Yu D et al. mGluR5 ablation in cortical glutamatergic neurons increases novelty-induced locomotion. PLoS One 2013; 8: e70415.
O’Tuathaigh CMP, Babovic D, O’Sullivan GJ, Clifford JJ, Tighe O, Croke DT et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience 2007; 147: 18–27.
Silverman JL, Yang M, Lord C, Crawley JN . Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 2010; 11: 490–502.
Madsen GF, Bilenberg N, Cantio C, Oranje B . Increased prepulse inhibition and sensitization of the startle reflex in autistic children. Autism Res 2013; 7: 94–103.
Braff DL, Geyer MA, Swerdlow NR . Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001; 156: 234–258.
Perry W, Minassian A, Lopez B, Maron L, Lincoln A . Sensorimotor gating deficits in adults with autism. Biol Psychiatry 2007; 61: 482–486.
Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R . Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46: 56–72.
Jones CA, Watson DJ, Fone KC . Animal models of schizophrenia. Br J Pharmacol 2011; 164: 1162–1194.
Woo TU, Whitehead RE, Melchitzky DS, Lewis DA . A subclass of prefrontal γ-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA 1998; 95: 5341–5346.
Zhang ZJ, Reynolds GP . A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophr Res 2002; 55: 1–10.
Gogolla N, LeBlanc J, Quast K, Südhof T, Fagiolini M, Hensch T . Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodevelop Disord 2009; 1: 172–181.
Kataoka Y, Kalanithi PS, Grantz H, Schwartz ML, Saper C, Leckman JF et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol 2010; 518: 277–291.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007; 445: 168–176.
Powell SB, Sejnowski TJ, Behrens MM . Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology 2012; 62: 1322–1331.
Tatard-Leitman VM, Jutzeler CR, Suh J, Saunders JA, Billingslea EN, Morita S et al. Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biol Psychiatry 2015; 77: 556–568.
Uhlhaas PJ, Singer W . Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci 2010; 11: 100–113.
Barr MS, Farzan F, Tran LC, Chen R, Fitzgerald PB, Daskalakis ZJ . Evidence for excessive frontal evoked gamma oscillatory activity in schizophrenia during working memory. Schizophr Res 2010; 121: 146–152.
Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci 2009; 29: 9481–9489.
Lee SH, Wynn JK, Green MF, Kim H, Lee KJ, Nam M et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophr Res 2006; 83: 111–119.
Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM . Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int J Psychophysiol 2011; 79: 55–63.
Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW . Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci 2009; 10: 85.
Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry 2007; 62: 1022–1029.
Rojas DC, Maharajh K, Teale P, Rogers SJ . Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry 2008; 8: 66.
Cornew L, Roberts TL, Blaskey L, Edgar JC . Resting-state oscillatory activity in autism spectrum disorders. J Autism Dev Disord 2012; 42: 1884–1894.
Broadbent NJ, Squire LR, Clark RE . Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA 2004; 101: 14515–14520.
Andersen JD, Pouzet B . Spatial Memory deficits induced by perinatal treatment of rats with PCP and reversal effect of D-serine. Neuropsychopharmacology 2004; 29: 1080–1090.
Harich S, Gross G, Bespalov A . Stimulation of the metabotropic glutamate 2/3 receptor attenuates social novelty discrimination deficits induced by neonatal phencyclidine treatment. Psychopharmacology 2007; 192: 511–519.
Brown MW, Warburton EC, Aggleton JP . Recognition memory: Material, processes, and substrates. Hippocampus 2010; 20: 1228–1244.
Christoffersen GR, Simonyi A, Schachtman TR, Clausen B, Clement D, Bjerre VK et al. MGlu5 antagonism impairs exploration and memory of spatial and non-spatial stimuli in rats. Behav Brain Res 2008; 191: 235–245.
Gittis AH, Kreitzer AC . Striatal microcircuitry and movement disorders. Trends Neurosci 2012; 35: 557–564.
Shepherd GM . Corticostriatal connectivity and its role in disease. Nat Rev Neurosci 2013; 14: 278–291.
Burguière E, Monteiro P, Feng G, Graybiel AM . Optogenetic Stimulation of Lateral Orbitofronto-Striatal Pathway Suppresses Compulsive Behaviors. Science 2013; 340: 1243–1246.
Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH et al. Development of a mouse test for repetitive, restricted behaviors: Relevance to autism. Behav Brain Res 2008; 188: 178–194.
Chao HT . Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 2010; 468: 263–269.
Werling DM, Geschwind DH . Sex differences in autism spectrum disorders. Curr Opin Neurol 2013; 26: 146–153.
Pop AS, Gomez-Mancilla B, Neri G, Willemsen R, Gasparini F . Fragile X syndrome: a preclinical review on metabotropic glutamate receptor 5 (mGluR5) antagonists and drug development. Psychopharmacology (Berl) 2014; 231: 1217–1226.
Chen L, Toth M . Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 2001; 103: 1043–1050.
Selby L, Zhang C, Sun Q-Q . Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett 2007; 412: 227–232.
Sohal VS . Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry 2012; 71: 1039–1045.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012; 62: 1574–1583.
Auclair A, Cotecchia S, Glowinski J, Tassin JP . D-amphetamine fails to increase extracellular dopamine levels in mice lacking alpha 1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci 2002; 22: 9150–9154.
Lodge DJ, Grace AA . Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotox Res 2008; 14: 97–104.
Lisman JE, Pi HJ, Zhang Y, Otmakhova NA . A thalamo-hippocampal-ventral tegmental area loop may produce the positive feedback that underlies the psychotic break in schizophrenia. Biol Psychiatry 2010; 68: 17–24.
Acknowledgements
This work was supported by NIH grants R01MH91407 and R01MH94670 to MMB, R01MH62527 to A Markou and HHMI to TJS. EAM was supported by NIH/NINDS grant K99NS080911. AP-D was a recipient of a Calouste Gulbenkian Foundation Fellowship. We thank Dr Stephen F Heinemann and Jian Xu for providing us the mGluR5-LoxP mouse line, Joseph Chambers for technical assistance with animal breeding, Dr William F Loomis, Dr James Kesby and Dr Kyongmi Um for their insightful comments and Michael Arends for editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
During the last 3 years, A Markou has received research contract support from Astra-Zeneca, Bristol-Myers-Squibb and Forest Laboratories, and honoraria from AbbVie, Germany.
Additional information
Supplementary Information accompanies the paper on the Molecular Psychiatry website
Supplementary information
Rights and permissions
About this article
Cite this article
Barnes, S., Pinto-Duarte, A., Kappe, A. et al. Disruption of mGluR5 in parvalbumin-positive interneurons induces core features of neurodevelopmental disorders. Mol Psychiatry 20, 1161–1172 (2015). https://doi.org/10.1038/mp.2015.113
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/mp.2015.113
This article is cited by
-
mGluR5 in hippocampal CA1 pyramidal neurons mediates stress-induced anxiety-like behavior
Neuropsychopharmacology (2023)
-
Montelukast reduces grey matter abnormalities and functional deficits in a mouse model of inflammation-induced encephalopathy of prematurity
Journal of Neuroinflammation (2022)
-
Auditory processing in rodent models of autism: a systematic review
Journal of Neurodevelopmental Disorders (2022)
-
An efficient rAAV vector for protein expression in cortical parvalbumin expressing interneurons
Scientific Reports (2022)
-
From bench to bedside: The mGluR5 system in people with and without Autism Spectrum Disorder and animal model systems
Translational Psychiatry (2022)