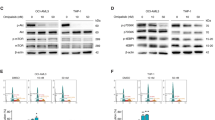
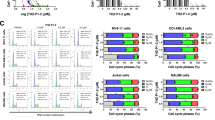
Abstract
Identification of agents that target human leukemia stem cells is an important consideration for the development of new therapies. The present study demonstrates that rocaglamide and silvestrol, closely related natural products from the flavagline class of compounds, are able to preferentially kill functionally defined leukemia stem cells, while sparing normal stem and progenitor cells. In addition to efficacy as single agents, flavaglines sensitize leukemia cells to several anticancer compounds, including front-line chemotherapeutic drugs used to treat leukemia patients. Mechanistic studies indicate that flavaglines strongly inhibit protein synthesis, leading to the reduction of short-lived antiapoptotic proteins. Notably though, treatment with flavaglines, alone or in combination with other drugs, yields a much stronger cytotoxic activity toward leukemia cells than the translational inhibitor temsirolimus. These results indicate that the underlying cell death mechanism of flavaglines is more complex than simply inhibiting general protein translation. Global gene expression profiling and cell biological assays identified Myc inhibition and the disruption of mitochondrial integrity to be features of flavaglines, which we propose contribute to their efficacy in targeting leukemia cells. Taken together, these findings indicate that rocaglamide and silvestrol are distinct from clinically available translational inhibitors and represent promising candidates for the treatment of leukemia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jordan CT, Guzman ML, Noble M . Cancer stem cells. N Engl J Med 2006; 355: 1253–1261.
Wang JC, Dick JE . Cancer stem cells: lessons from leukemia. Trends Cell Biol 2005; 15: 494–501.
Becker MW, Jordan CT . Leukemia stem cells in 2010: current understanding and future directions. Blood Rev 2011; 25: 75–81.
Lucas DM, Still PC, Perez LB, Grever MR, Kinghorn AD . Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Current Drug Targets 2010; 11: 812–822.
Kim S, Salim AA, Swanson SM, Kinghorn AD . Potential of cyclopenta[b]benzofurans from Aglaia species in cancer chemotherapy. Anti-cancer Agents Med Chem 2006; 6: 319–345.
Zhu JY, Lavrik IN, Mahlknecht U, Giaisi M, Proksch P, Krammer PH et al. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int J Cancer 2007; 121: 1839–1846.
Lucas DM, Edwards RB, Lozanski G, West DA, Shin JD, Vargo MA et al. The novel plant-derived agent silvestrol has B-cell selective activity in chronic lymphocytic leukemia and acute lymphoblastic leukemia in vitro and in vivo. Blood 2009; 113: 4656–4666.
Zhu JY, Giaisi M, Kohler R, Muller WW, Muhleisen A, Proksch P et al. Rocaglamide sensitizes leukemic T cells to activation-induced cell death by differential regulation of CD95L and c-FLIP expression. Cell Death Differ 2009; 16: 1289–1299.
Bleumink M, Kohler R, Giaisi M, Proksch P, Krammer PH, Li-Weber M . Rocaglamide breaks TRAIL resistance in HTLV-1-associated adult T-cell leukemia/lymphoma by translational suppression of c-FLIP expression. Cell Death Differ 2011; 18: 362–370.
Alinari L, Prince CJ, Edwards RB, Towns WH, Mani R, Lehman A et al. Dual targeting of the cyclin/Rb/E2F and mitochondrial pathways in mantle cell lymphoma with the translation inhibitor silvestrol. Clin Cancer Res 2012; 18: 4600–4611.
Hwang BY, Su BN, Chai H, Mi Q, Kardono LB, Afriastini JJ et al. Silvestrol and episilvestrol, potential anticancer rocaglate derivatives from Aglaia silvestris. J Org Chem 2004; 69: 3350–3358.
Malina A, Cencic R, Pelletier J . Targeting translation dependence in cancer. Oncotarget 2011; 2: 76–88.
Cencic R, Carrier M, Galicia-Vazquez G, Bordeleau ME, Sukarieh R, Bourdeau A et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, silvestrol. PLoS One 2009; 4: e5223.
Chambers JM, Lindqvist LM, Webb A, Huang DC, Savage GP, Rizzacasa MA . Synthesis of biotinylated episilvestrol: highly selective targeting of the translation factors eIF4AI/II. Org Lett 2013; 15: 1406–1409.
Sadlish H, Galicia-Vazquez G, Paris CG, Aust T, Bhullar B, Chang L et al. Evidence for a functionally relevant rocaglamide binding Site on the eIF4A-RNA complex. ACS Chem Biol 2013; 8: 1519–1527.
Grzmil M, Hemmings BA . Translation regulation as a therapeutic target in cancer. Cancer Res 2012; 72: 3891–3900.
Tamburini J, Green AS, Bardet V, Chapuis N, Park S, Willems L et al. Protein synthesis is resistant to rapamycin and constitutes a promising therapeutic target in acute myeloid leukemia. Blood 2009; 114: 1618–1627.
Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005; 105: 4163–4169.
Lagadinou ED, Ziros PG, Tsopra OA, Dimas K, Kokkinou D, Thanopoulou E et al. c-Jun N-terminal kinase activation failure is a new mechanism of anthracycline resistance in acute myeloid leukemia. Leukemia 2008; 22: 1899–1908.
Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 2013; 12: 329–341.
Bordeleau ME, Robert F, Gerard B, Lindqvist L, Chen SM, Wendel HG et al. Therapeutic suppression of translation initiation modulates chemosensitivity in a mouse lymphoma model. J Clin Invest 2008; 118: 2651–2660.
Lindqvist LM, Vikstrom I, Chambers JM, McArthur K, Ann Anderson M, Henley KJ et al. Translation inhibitors induce cell death by multiple mechanisms and Mcl-1 reduction is only a minor contributor. Cell Death Dis 2012; 3: e409.
Kim S, Hwang BY, Su BN, Chai H, Mi Q, Kinghorn AD et al. Silvestrol, a potential anticancer rocaglate derivative from Aglaia foveolata, induces apoptosis in LNCaP cells through the mitochondrial/apoptosome pathway without activation of executioner caspase-3 or -7. Anticancer Res 2007; 27: 2175–2183.
Lindqvist L, Pelletier J . Inhibitors of translation initiation as cancer therapeutics. Fut Med Chem 2009; 1: 1709–1722.
Recher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, Mas VM et al. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 2005; 105: 2527–2534.
Assouline S, Culjkovic B, Cocolakis E, Rousseau C, Beslu N, Amri A et al. Molecular targeting of the oncogene eIF4E in acute myeloid leukemia (AML): a proof-of-principle clinical trial with ribavirin. Blood 2009; 114: 257–260.
Chan S, Scheulen ME, Johnston S, Mross K, Cardoso F, Dittrich C et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol 2005; 23: 5314–5322.
Staehler M, Haseke N, Khoder W, Stief CG . Profile of temsirolimus in the treatment of advanced renal cell carcinoma. OncoTargets Ther 2010; 3: 191–196.
Kentsis A, Topisirovic I, Culjkovic B, Shao L, Borden KL . Ribavirin suppresses eIF4E-mediated oncogenic transformation by physical mimicry of the 7-methyl guanosine mRNA cap. Proc Natl Acad Sci USA 2004; 101: 18105–18110.
Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005; 435: 677–681.
Chiosis G, Kang Y, Sun W . Discovery and development of purine-scaffold Hsp90 inhibitors. Expert Opin Drug Discov 2008; 3: 99–114.
Wen J, You KR, Lee SY, Song CH, Kim DG . Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 2002; 277: 38954–38964.
Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 2010; 143: 313–324.
Lin CJ, Nasr Z, Premsrirut PK, Porco JA Jr, Hippo Y, Lowe SW et al. Targeting synthetic lethal interactions between Myc and the eIF4F complex impedes tumorigenesis. Cell Rep 2012; 1: 325–333.
Malona JA, Cariou K, Frontier AJ . Nazarov cyclization initiated by peracid oxidation: the total synthesis of (+/−)-rocaglamide. J Am Chem Soc 2009; 131: 7560–7561.
Gerard B, Sangji S, O'Leary DJ, Porco JA Jr. . Enantioselective photocycloaddition mediated by chiral Bronsted acids: asymmetric synthesis of the rocaglamides. J Am Chem Soc 2006; 128: 7754–7755.
Davey AE, Schaeffer MJ, Taylor RJK . Synthesis of the Novel antileukemic tetrahydrocyclopenta[b]benzofuran, rocaglamide. J Chem Soc Chem Commun 1991, 1137–1139.
Dobler MR, Bruce I, Cederbaum F, Cooke NG, Diorazio LJ, Hall RG et al. Total synthesis of (+/−)-rocaglamide and some aryl analogues. Tetrahedron Lett 2001; 42: 8281–8284.
Hongsen L, Fu B, Wang MA, Li N, Liu WJ, Xie ZQ et al. Total synthesis and biological activity of (+/−)-rocaglamide and its 2,3-di-epi analogue. Eur J Org Chem 2008, 1753–1758.
Trost BM, Greenspan PD, Yang BV, Saulnier MG . An unusual oxidative cyclization—a synthesis and absolute stereochemical assignment of (−)-rocaglamide. J Am Chem Soc 1990; 112: 9022–9024.
Saradhi UV, Gupta SV, Chiu M, Wang J, Ling Y, Liu Z et al. Characterization of silvestrol pharmacokinetics in mice using liquid chromatography-tandem mass spectrometry. AAPS J 2011; 13: 347–356.
Pan L, Kardono LB, Riswan S, Chai H, Carcache de Blanco EJ, Pannell CM et al. Isolation and characterization of minor analogues of silvestrol and other constituents from a large-scale re-collection of Aglaia foveolata. J Nat Prod 2010; 73: 1873–1878.
Jordan CT . Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia 2002; 16: 559–562.
Dick JE . Acute myeloid leukemia stem cells. Ann NY Acad Sci 2005; 1044: 1–5.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacobs HM et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011; 146: 904–917.
Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci USA 2011; 108: 16669–16674.
Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature 2011; 478: 524–528.
Silvera D, Formenti SC, Schneider RJ . Translational control in cancer. Nat Rev Cancer 2010; 10: 254–266.
Stumpf CR, Ruggero D . The cancerous translation apparatus. Curr Opin Genet Dev 2011; 21: 474–483.
Graff JR, Konicek BW, Vincent TM, Lynch RL, Monteith D, Weir SN et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest 2007; 117: 2638–2648.
Moerke NJ, Aktas H, Chen H, Cantel S, Reibarkh MY, Fahmy A et al. Small-molecule inhibition of the interaction between the translation initiation factors eIF4E and eIF4G. Cell 2007; 128: 257–267.
Acknowledgements
This work was supported by the Leukemia and Lymphoma Society (CTJ, 6230-11), Department of Defense (CTJ, W81XWH-07-1-0601), NY State Stem Cell Foundation (CTJ, C024964), National Institutes of Health (AF, RO1 GM079364), (ADK, PO1 CA125066) and a Wilmot-Roswell Park Collaborative Seed Grant. KPC was supported by American Cancer Society (PF-10-054-01-LIB) and Wilmot Cancer Center postdoctoral fellowships.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interests.
Additional information
Author contributions
KPC designed research, performed experiment, and wrote the paper; CTJ designed research and wrote the paper; MM, CC, EDL, RMR, MBB, LP, SJ, AF, MRG, ADK, JLL, MWB performed experiments and analyzed data.
Supplementary Information accompanies this paper on the Leukemia website
Supplementary information
Rights and permissions
About this article
Cite this article
Callahan, K., Minhajuddin, M., Corbett, C. et al. Flavaglines target primitive leukemia cells and enhance anti-leukemia drug activity. Leukemia 28, 1960–1968 (2014). https://doi.org/10.1038/leu.2014.93
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/leu.2014.93
This article is cited by
-
EIF4A inhibition targets bioenergetic homeostasis in AML MOLM-14 cells in vitro and in vivo and synergizes with cytarabine and venetoclax
Journal of Experimental & Clinical Cancer Research (2022)
-
Comparative phytochemistry of flavaglines (= rocaglamides), a group of highly bioactive flavolignans from Aglaia species (Meliaceae)
Phytochemistry Reviews (2022)
-
Flavagline analog FL3 induces cell cycle arrest in urothelial carcinoma cell of the bladder by inhibiting the Akt/PHB interaction to activate the GADD45α pathway
Journal of Experimental & Clinical Cancer Research (2018)