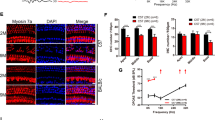
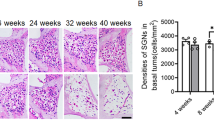
Abstract
Previously, we showed that age-related hearing loss (AHL) was delayed in C57BL6 mice overexpressing X-Linked Inhibitor of Apoptotic Protein (XIAP), and the delayed AHL was associated with attenuated hair cell (HC) loss in XIAP-overexpressing mice. Similar to other reports, the HC loss in aged mice was restricted to the basal turn in this previous study, and occurred slightly at the apical end of the cochlea, showing considerably less spread than the frequency region of hearing loss. In the present study, we examined whether and how AHL is related to the degeneration of neuronal innervation of the cochlea and whether the overexpression of XIAP exerts a protective effect against age-related degeneration in both afferent and efferent cochlear neurites. In contrast to HC loss, degeneration of both afferent and efferent neurites spread to the middle turns of the cochlea. Moreover, XIAP-overexpressing mice lost fewer HC afferent dendrites and efferent axons, as well as fewer spiral ganglion neurons between 3 and 14 months of age in comparison with wild-type littermates. The results suggest that age-related degeneration of cochlear neurites may be independent of HC loss. Further, the inhibition of apoptosis by XIAP appears to reduce degeneration of both afferent and efferent cochlear neurites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Garfinkle TJ, Saunders JC . Morphology of inner hair cell stereocilia in C57BL/6J mice as studied by scanning electron microscopy. Otolaryngol Head Neck Surg 1983; 91: 421–426.
Spongr VP, Flood DG, Frisina RD, Salvi RJ . Quantitative measure of hair cell loss in CBA and C57BL/6 mice throughout their life spans. J Acoust Soc Am 1997; 101: 3546–3553.
White JA, Burgess BJ, Hall RD, Nadol JB . Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res 2000; 141: 12–18.
Hequembourg S, Liberman MC . Spiral ligament pathology: a major aspect of age-related cochlear degeneration in C57BL/6 mice. J Assoc Res Otolaryngol 2001; 2: 118–129.
Shnerson A, Devigne C, Pujol R . Age-related changes in the C57BL/6J mouse cochlea. II. Ultrastructural findings. Brain Res 1981; 254: 77–88.
Wang J, Menchenton T, Yin S, Yu Z, Bance M, Morris DP et al. Over-expression of X-linked inhibitor of apoptosis protein slows presbycusis in C57BL/6J mice. Neurobiol Aging 2010; 31: 1238–1249.
Felder E, Schrott-Fischer A . Quantitative evaluation of myelinated nerve fibres and hair cells in cochleae of humans with age-related high-tone hearing loss. Hear Res 1995; 91: 19–32.
Keithley EM, Ryan AF, Woolf NK . Spiral ganglion cell density in young and old gerbils. Hear Res 1989; 38: 125–133.
Ryals BM, Eyck BT, Westbrook EW . Ganglion cell loss continues during hair cell regeneration. Hear Res 1989; 43: 81–90.
Stamataki S, Francis HW, Lehar M, May BJ, Ryugo DK . Synaptic alterations at inner hair cells precede spiral ganglion cell loss in aging C57BL/6J mice. Hear Res 2006; 221: 104–118.
Alam SA, Oshima T, Suzuki M, Kawase T, Takasaka T, Ikeda K . The expression of apoptosis-related proteins at the aged cochlea of Mongolian gerbils. Laryngoscope 2001; 111: 528–534.
Iwai H, Lee S, Inaba M, Sugiura K, Tomoda K, Yamashita T et al. Prevention of accelerated presbycusis by bone marrow transplantation in senescence-accelerated mice. Bone Marrow Transplant 2001; 28: 323–328.
Pickles JO . Mutation in mitochondrial DNA as a cause of presbyacusis. Audiol Neurootol 2004; 9: 23–33.
Spicer SS, Schulte BA . Spiral ligament pathology in quiet-aged gerbils. Hear Res 2002; 172: 172–185.
Tadros SF, D'Souza M, Zhu X, Frisina RD . Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis 2008; 13: 1303–1321.
Zheng Y, Ikeda K, Nakamura M, Takasaka T . Endonuclease cleavage of DNA in the aged cochlea of Mongolian gerbil. Hear Res 1998; 126: 11–18.
Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ . Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell 1993; 75: 241–251.
Hosokawa M . A higher oxidative status accelerates senescence and aggravates age-dependent disorders in SAMP strains of mice. Mech Ageing Dev 2002; 123: 1553–1561.
Someya S, Prolla TA . Mitochondrial oxidative damage and apoptosis in age-related hearing loss. Mech Ageing Dev 2010; 131: 480–486.
Usami S, Takumi Y, Fujita S, Shinkawa H, Hosokawa M . Cell death in the inner ear associated with aging is apoptosis? Brain Res 1997; 747: 147–150.
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC . Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 1999; 18: 5242–5251.
Deveraux QL, Stennicke HR, Salvesen GS, Reed JC . Endogenous inhibitors of caspases. J Clin Immunol 1999; 19: 388–398.
Deveraux QL, Takahashi R, Salvesen GS, Reed JC . X-linked IAP is a direct inhibitor of cell-death proteases. Nature 1997; 388: 300–304.
Liston P, Roy N, Tamai K, Lefebvre C, Baird S, Cherton-Horvat GF et al. Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 1996; 379: 349–353.
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R . X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem 2001; 276: 27058–27063.
Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY . A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res 1997; 114: 83–92.
Syka J . Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev 2002; 82: 601–636.
Wang J, Tymczyszyn N, Yu Z, Yin S, Bance M, Robertson GS . Overexpression of X-linked inhibitor of apoptosis protein protects against noise-induced hearing loss in mice. Gene Therapy 2011; 18: 560–568.
Kujawa SG, Liberman MC . Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci 2009; 29: 14077–14085.
Zilberstein Y, Liberman MC, Corfas G . Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci 2012; 32: 405–410.
Wang J, Powers NL, Hofstetter P, Trautwein P, Ding D, Salvi R . Effects of selective inner hair cell loss on auditory nerve fiber threshold, tuning and spontaneous and driven discharge rate. Hear Res 1997; 107: 67–82.
Ohlemiller KK, Gagnon PM . Apical-to-basal gradients in age-related cochlear degeneration and their relationship to "primary" loss of cochlear neurons. J Comp Neurol 2004; 479: 103–116.
Thiers FA, Nadol Jr JB, Liberman MC . Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J Assoc Res Otolaryngol 2008; 9: 477–489.
Ruan Q, Ao H, He J, Chen Z, Yu Z, Zhang R et al. Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology 2014; 40: 86–96.
Wilson JL, Henson MM, Henson Jr OW . Course and distribution of efferent fibers in the cochlea of the mouse. Hear Res 1991; 55: 98–108.
Schwartz AM, Parakkal M, Gulley RL . Postnatal development of spiral ganglion cells in the rat. Am J Anat 1983; 167: 33–41.
Dau J, Wenthold RJ . Immunocytochemical localization of neurofilament subunits in the spiral ganglion of normal and neomycin-treated guinea pigs. Hear Res 1989; 42: 253–263.
Fritzsch B . Fast axonal diffusion of 3000 molecular weight dextran amines. J Neurosci Methods 1993; 50: 95–103.
Kobbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S . Current concepts in neuroanatomical tracing. Prog. Neurobiol 2000; 62: 327–351.
Huang L, Thorne P, Houseley G, Montgometry J . Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development 2007; 134: 2925–2933.
Hafidi A . Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res 1998; 805: 181–190.
Hafidi A, Despres G, Romand R . Ontogenesis of type II spiral ganglion neurons during development: peripherin immunohistochemistry. Int J Dev Neurosci 1993; 11: 507–512.
Mou K, Adamson CL, Davis RL . Time-dependence and cell type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol 1998; 402: 129–139.
Schimmang T, Tan J, Muller M, Zimmermann U, Rohbock K, Kopschall I et al. Lack of Bdnf and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development 2000; 130: 4741–4750.
Acknowledgements
This study was supported by a grant-in-aid for Scientific Research from Shanghai Municipal Health Bureau (No. 2011-4144), the State Key Development Program for Basic Research of China (Grant no. 2011CB504503 and no. 2012CB967903), the National Science Foundation for Distinguished Young Scholars of China (Grant no. 30925035) and the Canadian Institute of Health Research (MOP79452). The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/D1ntYv.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Ruan, Q., Zeng, S., Liu, A. et al. Overexpression of X-Linked Inhibitor of Apoptotic Protein (XIAP) reduces age-related neuronal degeneration in the mouse cochlea. Gene Ther 21, 967–974 (2014). https://doi.org/10.1038/gt.2014.77
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2014.77