Abstract
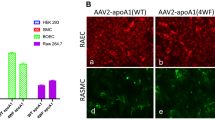
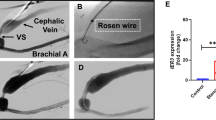
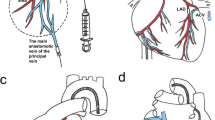
Coronary artery disease represents the leading cause of mortality in the developed world. Percutaneous coronary intervention involving stent placement remains disadvantaged by restenosis or thrombosis. Vascular gene therapy-based methods may be approached, but lack a vascular gene delivery vector. We report a safe and efficient long-term transduction of rat carotid vessels after balloon injury intervention with a translational optimized AAV2.5 vector. Compared with other known adeno-associated virus (AAV) serotypes, AAV2.5 demonstrated the highest transduction efficiency of human coronary artery vascular smooth muscle cells (VSMCs) in vitro. Local delivery of AAV2.5-driven transgenes in injured carotid arteries resulted in transduction as soon as day 2 after surgery and persisted for at least 30 days. In contrast to adenovirus 5 vector, inflammation was not detected in AAV2.5-transduced vessels. The functional effects of AAV2.5-mediated gene transfer on neointimal thickening were assessed using the sarco/endoplasmic reticulum Ca2+ ATPase isoform 2a (SERCA2a) human gene, known to inhibit VSMC proliferation. At 30 days, human SERCA2a messenger RNA was detected in transduced arteries. Morphometric analysis revealed a significant decrease in neointimal hyperplasia in AAV2.5–SERCA2a-transduced arteries: 28.36±11.30 (n=8) vs 77.96±24.60 (n=10) μm2, in AAV2.5–green fluorescent protein-infected, P<0.05. In conclusion, AAV2.5 vector can be considered as a promising safe and effective vector for vascular gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 2010; 121: 948–954.
Robertson KE, McDonald RA, Oldroyd KG, Nicklin SA, Baker AH . Prevention of coronary in-stent restenosis and vein graft failure: does vascular gene therapy have a role? Pharmacol Ther 2012; 136: 23–34.
Rutanen J, Puhakka H, Yla-Herttuala S . Post-intervention vessel remodeling. Gene Therapy 2002; 9: 1487–1491.
Curcio A, Torella D, Indolfi C . Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting: approach to therapy. Circ J 2011; 75: 1287–1296.
Gaffney MM, Hynes SO, Barry F, O'Brien T . Cardiovascular gene therapy: current status and therapeutic potential. Br J Pharmacol 2007; 152: 175–188.
Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail 2009; 15: 171–181.
Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure. Circulation 2012; 124: 304–313.
Lipskaia L, del Monte F, Capiod T, Yacoubi S, Hadri L, Hours M et al. Sarco/endoplasmic reticulum Ca2+-ATPase gene transfer reduces vascular smooth muscle cell proliferation and neointima formation in the rat. Circ Res 2005; 97: 488–495.
Lipskaia L, Hadri L, Le Prince P, Esposito B, Atassi F, Liang L et al. SERCA2a gene transfer prevents intimal proliferation in an organ culture of human internal mammary artery. Gene Therapy 2012, e-pub ahead of print 5 July 2012 doi:10.1038/gt.2012.50.
Merlet E, Lipskaia L, Marchand A, Hadri L, Mougenot N, Atassi F et al. A calcium-sensitive promoter construct for gene therapy. Gene Therapy 2013; 20: 248–254.
Bobe R, Hadri L, Lopez JJ, Sassi Y, Atassi F, Karakikes I et al. SERCA2a controls the mode of agonist-induced intracellular Ca2+ signal, transcription factor NFAT and proliferation in human vascular smooth muscle cells. J Mol Cell Cardiol 2011; 50: 621–633.
White K, Nicklin SA, Baker AH . Novel vectors for in vivo gene delivery to vascular tissue. Expert Opin Biol Ther 2007; 7: 809–821.
Gruchala M, Bhardwaj S, Pajusola K, Roy H, Rissanen TT, Kokina I et al. Gene transfer into rabbit arteries with adeno-associated virus and adenovirus vectors. J Gene Med 2004; 6: 545–554.
Schulick AH, Dong G, Newman KD, Virmani R, Dichek DA . Endothelium-specific in vivo gene transfer. Circ Res 1995; 77: 475–485.
Yang Y, Nunes FA, Berencsi K, Furth EE, Gonczol E, Wilson JM . Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA 1994; 91: 4407–4411.
Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T . Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation 1993; 88: 2838–2848.
Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T . Efficient gene transfer into myocardium by direct injection of adenovirus vectors. Circ Res 1993; 73: 1202–1207.
Hartman ZC, Appledorn DM, Amalfitano A . Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res 2008; 132: 1–14.
Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA et al. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity 2009; 31: 110–121.
Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, Waddington SN et al. Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors. Blood 2010; 116: 2656–2664.
Wu Z, Asokan A, Samulski RJ . Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther 2006; 14: 316–327.
Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS et al. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 2012; 20: 443–455.
Summerford C, Samulski RJ . Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998; 72: 1438–1445.
Gnatenko D, Arnold TE, Zolotukhin S, Nuovo GJ, Muzyczka N, Bahou WF . Characterization of recombinant adeno-associated virus-2 as a vehicle for gene delivery and expression into vascular cells. J Investig Med 1997; 45: 87–98.
Maeda Y, Ikeda U, Ogasawara Y, Urabe M, Takizawa T, Saito T et al. Gene transfer into vascular cells using adeno-associated virus (AAV) vectors. Cardiovasc Res 1997; 35: 514–521.
Richter M, Iwata A, Nyhuis J, Nitta Y, Miller AD, Halbert CL et al. Adeno-associated virus vector transduction of vascular smooth muscle cells in vivo. Physiol Genomics 2000; 2: 117–127.
Vassalli G, Bueler H, Dudler J, von Segesser LK, Kappenberger L . Adeno-associated virus (AAV) vectors achieve prolonged transgene expression in mouse myocardium and arteries in vivo: a comparative study with adenovirus vectors. Int J Cardiol 2003; 90: 229–238.
Chen S, Kapturczak M, Loiler SA, Zolotukhin S, Glushakova OY, Madsen KM et al. Efficient transduction of vascular endothelial cells with recombinant adeno-associated virus serotype 1 and 5 vectors. Hum Gene Ther 2005; 16: 235–247.
Zabner J, Seiler M, Walters R, Kotin RM, Fulgeras W, Davidson BL et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol 2000; 74: 3852–3858.
Sen S, Conroy S, Hynes SO, McMahon J, O'Doherty A, Bartlett JS et al. Gene delivery to the vasculature mediated by low-titre adeno-associated virus serotypes 1 and 5. J Gene Med 2008; 10: 143–151.
Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med 2010; 363: 1429–1437.
Indolfi C, Torella D, Coppola C, Stabile E, Esposito G, Curcio A et al. Rat carotid artery dilation by PTCA balloon catheter induces neointima formation in presence of IEL rupture. Am J Physiol Heart Circ Physiol 2002; 283: H760–H767.
Lipskaia L, Pourci ML, Delomenie C, Combettes L, Goudouneche D, Paul JL et al. Phosphatidylinositol 3-kinase and calcium-activated transcription pathways are required for VLDL-induced smooth muscle cell proliferation. Circ Res 2003; 92: 1115–1122.
Karpurapu M, Wang D, Van Quyen D, Kim TK, Kundumani-Sridharan V, Pulusani S et al. Cyclin D1 is a bona fide target gene of NFATc1 and is sufficient in the mediation of injury-induced vascular wall remodeling. J Biol Chem 2010; 285: 3510–3523.
Rolling F, Nong Z, Pisvin S, Collen D . Adeno-associated virus-mediated gene transfer into rat carotid arteries. Gene Therapy 1997; 4: 757–761.
Work LM, Nicklin SA, Brain NJ, Dishart KL, Von Seggern DJ, Hallek M et al. Development of efficient viral vectors selective for vascular smooth muscle cells. Mol Ther 2004; 9: 198–208.
Nicklin SA, Buening H, Dishart KL, de Alwis M, Girod A, Hacker U et al. Efficient and selective AAV2-mediated gene transfer directed to human vascular endothelial cells. Mol Ther 2001; 4: 174–181.
White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED et al. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation 2004; 109: 513–519.
Johnson JS, Li C, DiPrimio N, Weinberg MS, McCown TJ, Samulski RJ . Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol 2010; 84: 8888–8902.
Hadri L, Bobe R, Kawase Y, Ladage D, Ishikawa K, Atassi F et al. SERCA2a gene transfer enhances eNOS expression and activity in endothelial cells. Mol Ther 2010; 18: 1284–1292.
Jang JH, Schaffer DV, Shea LD . Engineering biomaterial systems to enhance viral vector gene delivery. Mol Ther 2011; 19: 1407–1415.
Wang K, Kessler PD, Zhou Z, Penn MS, Forudi F, Zhou X et al. Local adenoviral-mediated inducible nitric oxide synthase gene transfer inhibits neointimal formation in the porcine coronary stented model. Mol Ther 2003; 7: 597–603.
Fishbein I, Alferiev I, Bakay M, Stachelek SJ, Sobolewski P, Lai M et al. Local delivery of gene vectors from bare-metal stents by use of a biodegradable synthetic complex inhibits in-stent restenosis in rat carotid arteries. Circulation 2008; 117: 2096–2103.
Uchida Y, Uchida Y, Matsuyama A, Koga A, Kanai M, Sakurai T . Formation of web- and membrane-like structures on the edges of bare-metal coronary stents. Circ J 2010; 74: 1830–1836.
Ueda M, Becker AE, Naruko T, Kojima A . Smooth muscle cell de-differentiation is a fundamental change preceding wound healing after percutaneous transluminal coronary angioplasty in humans. Coron Artery Dis 1995; 6: 71–81.
Lipskaia L, Limon I, Bobe R, Hajjar RJ . Calcium cycling in synthetic and contractile phasic or tonic vascular smooth muscle cells. In: Sugi H (ed) Current Basic and Pathological Approaches to the Function of Muscle Cells and Tissues - From Molecules to Humans. InTech, 2012.
Tanner FC, Meier P, Greutert H, Champion C, Nabel EG, Luscher TF . Nitric oxide modulates expression of cell cycle regulatory proteins: a cytostatic strategy for inhibition of human vascular smooth muscle cell proliferation. Circulation 2000; 101: 1982–1989.
Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM . Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Therapy 2008; 15: 1550–1557.
Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol 2008; 51: 1112–1119.
Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail 2008; 14: 355–367.
Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G . Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. Faseb J 2003; 17: 1183–1185.
Morishita R . Lessons from human arteries: how to design a gene therapy strategy for treatment of cardiovascular disease. Circ Res 1998; 82: 1349–1351.
Rekhter MD, Simari RD, Work CW, Nabel GJ, Nabel EG, Gordon D . Gene transfer into normal and atherosclerotic human blood vessels. Circ Res 1998; 82: 1243–1252.
Janssens SP . Applied gene therapy in preclinical models of vascular injury. Curr Atheroscler Rep 2003; 5: 186–190.
Laitinen M, Hartikainen J, Hiltunen MO, Eranen J, Kiviniemi M, Narvanen O et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum Gene Ther 2000; 11: 263–270.
Hedman M, Hartikainen J, Syvanne M, Stjernvall J, Hedman A, Kivela A et al. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT). Circulation 2003; 107: 2677–2683.
Kutryk MJ, Foley DP, van den Brand M, Hamburger JN, van der Giessen WJ, deFeyter PJ et al. Local intracoronary administration of antisense oligonucleotide against c-myc for the prevention of in-stent restenosis: results of the randomized investigation by the Thoraxcenter of antisense DNA using local delivery and IVUS after coronary stenting (ITALICS) trial. J Am Coll Cardiol 2002; 39: 281–287.
Kipshidze N, Iversen P, Overlie P, Dunlap T, Titus B, Lee D et al. First human experience with local delivery of novel antisense AVI-4126 with Infiltrator catheter in de novo native and restenotic coronary arteries: 6-month clinical and angiographic follow-up from AVAIL study. Cardiovasc Revasc Med 2007; 8: 230–235.
del Monte F, Harding SE, Schmidt U, Matsui T, Kang ZB, Dec GW et al. Restoration of contractile function in isolated cardiomyocytes from failing human hearts by gene transfer of SERCA2a. Circulation 1999; 100: 2308–2311.
Eggermont JA, Wuytack F, Verbist J, Casteels R . Expression of endoplasmic-reticulum Ca2(+)-pump isoforms and of phospholamban in pig smooth-muscle tissues. Biochem J 1990; 271: 649–653.
Keuylian Z, de Baaij JH, Glorian M, Rouxel C, Merlet E, Lipskaia L et al. The Notch pathway attenuates interleukin 1beta (IL1beta)-mediated induction of adenylyl cyclase 8 (AC8) expression during vascular smooth muscle cell (VSMC) trans-differentiation. J Biol Chem 2012; 287: 24978–24989.
Acknowledgements
This work was supported by AHA SDG 0930116N (LL), by NIH 1K01HL103176 (LH), by Leducq Foundation through the Caerus network (05 CVD 03 to AML and RJH), by NIH R01 HL078691, HL057263, HL071763, HL080498 and HL083156 (RJH).
Author contributions
A-ML, SWJMP, LL and RJH contributed to the conception, design, analysis and interpretation of all data; drafting the manuscript, revising it critically for important intellectual content and final approval of the manuscript. RJS and SWJMP designed and produced virus vectors. NM, A-ML, LL, EM, ZK and JC performed the in vivo gene transfer. A-ML, LL, EM and FA performed immunohistochemistry and morphometry experiments and the analysis of results. LL, EM and AM performed the in vitro data. LL, IK, RB and IL contributed the quantitative reverse transcription-PCR analysis. LH led the fluorescence-activated cell sorting analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Gene Therapy website
Rights and permissions
About this article
Cite this article
Lompré, AM., Hadri, L., Merlet, E. et al. Efficient transduction of vascular smooth muscle cells with a translational AAV2.5 vector: a new perspective for in-stent restenosis gene therapy. Gene Ther 20, 901–912 (2013). https://doi.org/10.1038/gt.2013.13
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2013.13
Keywords
This article is cited by
-
Inhibition of Neointima Hyperplasia, Inflammation, and Reactive Oxygen Species in Balloon-Injured Arteries by HVJ Envelope Vector-Mediated Delivery of Superoxide Dismutase Gene
Translational Stroke Research (2019)
-
Stent-based delivery of adeno-associated viral vectors with sustained vascular transduction and iNOS-mediated inhibition of in-stent restenosis
Gene Therapy (2017)
-
Inhalable delivery of AAV-based MRP4/ABCC4 silencing RNA prevents monocrotaline-induced pulmonary hypertension
Molecular Therapy - Methods & Clinical Development (2015)