Abstract
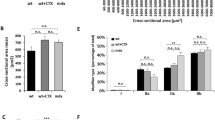
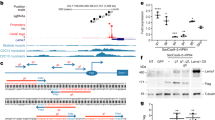
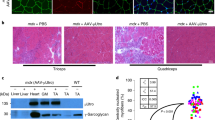
In utero gene therapy for genetic diseases, such as muscular dystrophies, offers potential advantages over postnatal treatment including vector delivery at the earliest point in the disease and treatment prior to full maturation of the immune system. This study examines in utero gene delivery of full-length murine dystrophin to the murine mdx model for Duchenne muscular dystrophy using a high-capacity adenoviral vector. We examined dystrophin expression, spread of vector, morphology and specific force production of the tibialis anterior muscle 9 weeks after intramuscular in utero injection. Recombinant dystrophin was expressed in the hindlimb muscles, with the majority of animals having expression in two muscles of the injected hindlimb. The dystrophin–glycoprotein complex was restored in those muscle fibers expressing recombinant dystrophin. Analysis of the percentage of dystrophin-expressing muscle fibers with centrally placed nuclei revealed effective protection from cycles of degeneration and regeneration normally seen in muscle fibers lacking dystrophin. However, due to low levels of muscle gene transfer, further advances in the efficiency of adenoviral vector-mediated gene delivery would be required for clinical applications of in utero gene therapy for primary myopathies such as Duchenne muscular dystrophy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Engel AG, Ozawa E . Dystrophinopathies. In: Engel AG, Franzini-Armstrong C (eds). Myology 2004: McGraw-Hill Book Company: New York, NY. Vol. 2, pp 961–1025.
Hoffman EP, Brown Jr RH, Kunkel LM . Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 1987; 51: 919–928.
Clemens PR, Fenwick RG, Chamberlain JS, Gibbs RA, de Andrade M, Chakraborty R et al. Carrier detection and prenatal diagnosis in Duchenne and Becker muscular dystrophy families, using dinucleotide repeat polymorphism. Am J Hum Genet 1991; 49: 951–960.
Evans MI, Farrell SA, Greb A, Ray P, Johnson MP, Hoffman EP . In utero fetal muscle biopsy for the diagnosis of Duchenne muscular-dystrophy in a female fetus suddenly at risk. Am J Med Genet 1993; 46: 309–312.
Evans MI, Hoffman EP, Cadrin C, Johnson MP, Quintero RA, Golbus MS . Fetal muscle biopsy—collaborative experience with varied indications. Obstet Gynecol 1994; 84: 913–917.
Nevo Y, Shomrat R, Yaron Y, Orr-Urtreger A, Harel S, Legum C . Fetal muscle biopsy as a diagnostic tool in Duchenne muscular dystrophy. Prenatal Diag 1999; 19: 921–926.
Blake DJ, Weir A, Newey SE, Davies KE . Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physio Rev 2002; 82: 291–329.
Roig M, Roma J, Fargas A, Munell F . Longitudinal pathologic study of the gastrocnemius muscle group in mdx mice. Acta Neurol 2004; 107: 27–34.
Bulfield G, Siller WG, Wight PAL, Moore KJ . X-chromosome linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci USA 1984; 81: 1189–1192.
Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ . The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 1989; 244: 1578–1580.
Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV, Faulkner JA . Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol 2001; 535: 591–600.
Jiang Z, Schiedner G, Gilchrist SC, Kochanek S, Clemens PR . CTLA4Ig delivered by high-capacity adenoviral vector induces stable expression of dystrophin in mdx mouse muscle. Gene Therapy 2004; 11: 1453–1461.
Chen HH, Mack LM, Kelly R, Ontell M, Kochanek S, Clemens PR . Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc Natl Acad Sci USA 1997; 94: 1645–1650.
Kochanek S, Clemens PR, Mitani K, Chen HH, Chan S, Caskey CT . A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc Natl Acad Sci USA 1996; 93: 5731–5736.
Phelps SF, Hauser MA, Cole NM, Rafael JA, Hinkle RT, Faulkner JA et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet 1995; 4: 1251–1258.
Wigmore PM, Dunglison GF . The generation of fiber diversity during myogenesis. Int J Dev Bio 1998; 42: 117–125.
Nalbantoglu J, Pari G, Karpati G, Holland PC . Expression of the primary coxsackie and adenovirus receptor is downregulated during skeletal muscle maturation and limits the efficacy of adenovirus-mediated gene delivery to muscle cells. Hum Gene Ther 1999; 10: 1009–1019.
Bilbao R, Reay DP, Hughes T, Biermann V, Volpers C, Goldberg L et al. Fetal muscle gene transfer is not enhanced by an RGD capsid modification to high-capacity adenoviral vectors. Gene Therapy 2003; 10: 1821–1829.
Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R et al. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 2005; 65: 826–834.
Waddington SN, Buckley SMK, Nivsarkar M, Jezzard S, Schneider H, Dahse T et al. In utero gene transfer of human factor IX to fetal mice can induce postnatal tolerance of the exogenous clotting factor. Blood 2003; 101: 1359–1366.
Matsumura K, Lee CC, Caskey CT, Campbell KP . Restoration of dystrophin-associated proteins in skeletal-muscle of mdx mice transgenic for dystrophin gene. FEBS Lett 1993; 320: 276–280.
Acsadi G, Jani A, Massie B, Simoneau M, Holland P, Blaschuk K et al. A differential efficiency of adenovirus-mediated in vivo gene-transfer into skeletal-muscle cells of different maturity. Hum Mol Genet 1994; 3: 579–584.
Clemens PR, Kochanek S, Sunada Y, Chan S, Chen HH, Campbell KP et al. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Therapy 1996; 3: 965–972.
DelloRusso C, Scott JM, Hartigan-O'Connor D, Salvatori G, Barjot C, Robinson AS et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci USA 2002; 99: 12979–12984.
Huard J, Feero WG, Watkins SC, Hoffman EP, Rosenblatt DJ, Glorioso JC . The basal lamina is a physical barrier to herpes simplex virus-mediated gene delivery to mature muscle fibers. J Virol 1996; 70: 8117–8123.
Dudley RWR, Lu YF, Gilbert R, Matecki S, Nalbantoglu J, Petrof BJ et al. Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum Gene Ther 2004; 15: 145–156.
Gilbert R, Dudley RWR, Liu AB, Petrof BJ, Nalbantoglu J, Karpati G . Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum Mol Genet 2003; 12: 1287–1299.
Bouchard S, MacKenzie TC, Radu AP, Hayashi S, Peranteau WH, Chirmule N et al. Long-term transgene expression in cardiac and skeletal muscle following fetal administration of adenoviral or adeno-associated viral vectors in mice. J Gene Med 2003; 5: 941–950.
Bilbao R, Reay DP, Li J, Xiao X, Clemens PR . Patterns of gene expression from in utero delivery of adenoviral-associated vector serotype 1. Hum Gene Ther 2005; 16: 678–684.
MacKenzie TC, Kobinger GP, Louboutin JP, Radu A, Javazon EH, Sena-Esteves M et al. Transduction of satellite cells after prenatal intramuscular administration of lentiviral vectors. J Gene Med 2005; 7: 50–58.
MacKenzie TC, Kobinger GP, Kootstra NA, Radu A, Sena-Esteves M, Bouchard S et al. Efficient transduction of liver and muscle after in utero injection of lentiviral vectors with different pseudotypes. Mol Ther 2002; 6: 349–358.
Gregory LG, Waddington SN, Holder MV, Mitrophanous KA, Buckley SMK, Mosley KL et al. Highly efficient EIAV-mediated in utero gene transfer and expression in the major muscle groups affected by Duchenne muscular dystrophy. Gene Therapy 2004; 11: 1117–1125.
Hauser MA, Robinson A, Hartigan-O'Connor D, Williams-Gregory D, Buskin JN, Apone S et al. Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol Ther 2000; 2: 16–25.
Bilbao R, Reay DP, Wu E, Zheng H, Biermann V, Kochanek S et al. Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero. Gene Therapy 2005; 12: 39–47.
Watchko J, O'Day T, Wang B, Zhou LQ, Tang Y, Li J et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther 2002; 13: 1451–1460.
Lu QL, Partridge TA . A new blocking method for application of murine monoclonal antibody to mouse tissue sections. J Histochem Cytochem 1998; 46: 977–983.
Acknowledgements
We thank Merck & Co. Inc., for helper virus H14 and 293Cre4 cells used to rescue and propagate AdmDys vector. We also acknowledge the laboratory of Dr Stefan Kochanek (University of Ulm, Ulm, Germany) for development of the AdmDys HC-Ad vector. This work was supported by Grant P01 AR45925 (PRC) from the NIH and grants from the Muscular Dystrophy Association (PRC). This material is also the result of work supported by VA resources (VA Healthcare System, Pittsburgh, PA, USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reay, D., Bilbao, R., Koppanati, B. et al. Full-length dystrophin gene transfer to the mdx mouse in utero. Gene Ther 15, 531–536 (2008). https://doi.org/10.1038/gt.2008.8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gt.2008.8
Keywords
This article is cited by
-
Mechanistic Insights into Factor VIII Immune Tolerance Induction via Prenatal Cell Therapy in Hemophilia A
Current Stem Cell Reports (2019)
-
In Utero Gene Therapy and Genome Editing
Current Stem Cell Reports (2018)
-
In utero stem cell transplantation and gene therapy: rationale, history, and recent advances toward clinical application
Molecular Therapy - Methods & Clinical Development (2016)
-
Improvement of the mdx mouse dystrophic phenotype by systemic in utero AAV8 delivery of a minidystrophin gene
Gene Therapy (2010)