Abstract
Background/Objectives:
The antiestrogen, Raloxifene (Ral) is an effective breast cancer chemopreventive agent. Omega-3 fatty acids (n-3FA) may inhibit mammary carcinogenesis. On the basis of their mechanisms of action, we test the hypothesis that a combination of n-3FA and Ral may be superior in reducing select biomarkers of breast cancer risk in women.
Subjects/Methods:
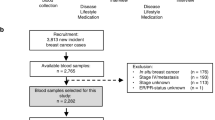
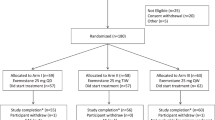
Postmenopausal women at increased risk for breast cancer (breast density ⩾25%) were randomized to: (1) no intervention; (2) Ral 60 mg; (3) Ral 30 mg; (4) n-3FA (Lovaza) 4 g and (5) Lovaza 4 g+Ral 30 mg for 2 years. Reduction in breast density is the primary end point of the study. We report preliminary data on feasibility, compliance and changes in secondary end points related to IGF-I signaling, estrogen metabolism, oxidative stress and inflammation in the first group of 46 women who completed 1 year of the study.
Results:
All interventions were well tolerated with excellent compliance (96±1% overall) by pill count and also supported by the expected rise in both serum n-3FA and n-3FA/Omega-6 fatty acids (n-6FA) ratio in women randomized to groups 4 and 5 (P<0.05). Lovaza decreased serum triglycerides and increased high-density lipoprotein (HDL) cholesterol compared with control (P<0.05 for both). Ral reduced serum IGF-1 in a dose-dependent manner (P<0.05) while Lovaza did not. Lovaza had no effect on IGF-1 or IGFBP-3. None of the other biomarkers were affected by our treatment.
Conclusion:
The combination of Lovaza and Ral is a feasible strategy that may be recommended in future breast cancer chemoprevention trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Signori C, El-Bayoumy K, Russo J, Thompson HJ, Richie JP, Hartman TJ et al. Chemoprevention of breast cancer by fish oil in preclinical models: trials and tribulations. Cancer Res 2011; 71: 6091–6096.
Manni A, Xu H, Washington S, Aliaga C, Cooper T, Richie JP et al. The impact of fish oil on the chemopreventive efficacy of tamoxifen against development of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Cancer Prev Res 2010; 3: 322–330.
Manni A, Richie JP, Xu H, Washington S, Aliaga C, Cooper TK et al. Effects of fish oil and Tamoxifen on preneoplastic lesion development and biomarkers of oxidative stress in the early stages of N-methyl-N-nitrosourea-induced rat mammary carcinogenesis. Int J Oncol 2011; 39: 1153–1164.
Manni A, Xu H, Washington S, Aliaga C, Das A, Cooper T et al. The effects of Tamoxifen and fish oil on mammary carcinogenesis in polyoma middle T transgenic mice. Horm Cancer 2011; 2: 249–259.
Boyd NF, Martin LJ, Rommens JM, Paterson AD, Minkin S, Yaffe MJ et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol 2009; 472: 343–360.
Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol 2001; 154: 1089–1099.
Miller PE, Lazarus P, Lesko SM, Muscat JE, Harper G, Cross AJ et al. Diet index-based and empirically derived dietary patterns are associated with colorectal cancer risk. J Nutr 2010; 140: 1267–1273.
Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395.
Slot C . Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest 1965; 17: 381–387.
Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998; 90: 1371–1388.
Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006; 295: 2727–2741.
Ropka ME, Keim J, Philbrick JT . Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol 2010; 28: 3090–3095.
Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN . Prevalence of tamoxifen use for breast cancer chemoprevention among U.S. women. Cancer Epidemiol Biomarkers Prev 2010; 19: 443–446.
Ruxton CH, Reed SC, Simpson MJ, Millington KJ . The health benefits of omega-3 polyunsaturated fatty acids: a review of the evidence. J Hum Nutr Diet 2007; 20: 275–285.
Yee LD, Lester JL, Cole RM, Richardson JR, Hsu JC, Li Y et al. Omega-3 fatty acid supplements in women at high risk of breast cancer have dose-dependent effects on breast adipose tissue fatty acid composition. Am J Clin Nutr 2010; 91: 1185–1194.
Harris WS . n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997; 65 (5 Suppl), 1645S–1654SS.
Skulas-Ray AC, Kris-Etherton PM, Harris WS, Vanden Heuvel JP, Wagner PR, West SG . Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nutr 2011; 93: 243–252.
Lee AV . YD. Role of the IGF System. In Breast Cancer Proliferation and Progression. Manni A (ed)., Humana Press, Inc.: Totowa, NJ, 1999.
Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M . Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004; 363: 1346–1353.
Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet 1998; 351: 1393–1396.
Torrisi R, Baglietto L, Johansson H, Veronesi G, Bonanni B, Guerrieri-Gonzaga A et al. Effect of raloxifene on IGF-I and IGFBP-3 in postmenopausal women with breast cancer. Br J Cancer 2001; 85: 1838–1841.
Oleksik AM, Duong T, Pliester N, Asma G, Popp-Snijders C, Lips P . Effects of the selective estrogen receptor modulator, raloxifene, on the somatotropic axis and insulin-glucose homeostasis. J Clin Endocrinol Metab 2001; 86: 2763–2768.
Eliassen AH, Missmer SA, Tworoger SS, Hankinson SE . Circulating 2-hydroxy- and 16alpha-hydroxy estrone levels and risk of breast cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2008; 17: 2029–2035.
Osborne MPKR, Hershcopf RJ, Bradlow HL, Kourides IA, Williams WR, Rosen PP et al. Omega-3 fatty acids: modulation of estrogen metabolism and potential for breast cancer prevention. Cancer Investigation 1988; 6: 629–631.
Rossner P, Gammon MD, Terry MB, Agrawal M, Zhang FF, Teitelbaum SL et al. Relationship between urinary 15-F2t-isoprostane and 8-oxodeoxyguanosine levels and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2006; 15: 639–644.
Acknowledgements
We thank Glaxo Smith Kline and Eli-Lilly for their generous supply of Lovaza and Raloxifene, respectively. This work was supported by Susan G Komen for the Cure Grant no. KG081632.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Signori, C., DuBrock, C., Richie, J. et al. Administration of omega-3 fatty acids and Raloxifene to women at high risk of breast cancer: interim feasibility and biomarkers analysis from a clinical trial. Eur J Clin Nutr 66, 878–884 (2012). https://doi.org/10.1038/ejcn.2012.60
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2012.60
Keywords
This article is cited by
-
Omega-3 fatty acids for breast cancer prevention and survivorship
Breast Cancer Research (2015)
-
Oxidized derivative of docosahexaenoic acid preferentially inhibit cell proliferation in triple negative over luminal breast cancer cells
In Vitro Cellular & Developmental Biology - Animal (2015)
-
n-3 PUFAs: an Elixir in Prevention of Colorectal Cancer
Current Colorectal Cancer Reports (2015)
-
Ratio of n-3/n-6 PUFAs and risk of breast cancer: a meta-analysis of 274135 adult females from 11 independent prospective studies
BMC Cancer (2014)
-
Omega-3 fatty acids: physiology, biological sources and potential applications in supportive cancer care
Phytochemistry Reviews (2014)