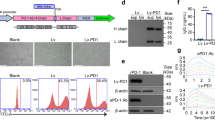
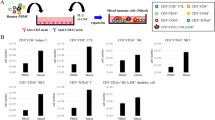
Abstract
Adoptive cell-transfer therapy (ACT) has been reported to suppress growing tumors and to overcome tumor escape in animal models. As a candidate ACT effector, γ9δ2T cells can be activated and expanded in vitro and in vivo and display strong antitumor activity against colorectal, lung, prostate, ovarian and renal cell carcinomas. However, it is difficult to obtain a large enough number of γδT cells to meet the need for immunotherapy that can overcome the cancer patients' immune suppressive tumor microenvironment. In previous studies, our lab confirmed that γ9δ2T cells recognized tumor cells via the CDR3δ region of the γδ-T-cell receptor (TCR). We constructed full-length human peripheral blood mononuclear cell (PBMC)-derived γ9 and δ2 chains in which the CDR3 region was replaced by an ovarian epithelial carcinoma (OEC)-derived CDR3. We transferred the CDR3δ-grafted γ9δ2TCR into peripheral blood lymphocytes (PBLs) to develop genetically modified γ9δ2T cells. In vitro studies have shown that these CDR3δ-grafted γ9δ2T cells can produce cytokines after stimulation with tumor cell extracts and exhibit cytotoxicity towards tumor cells, including human OEC and cervical adenocarcinoma. CDR3δ-grafted γ9δ2T cells adoptively transferred into nude mice bearing a human OEC cell line demonstrated significant antitumor effects. These results indicate that CDR3δ-grafted γ9δ2T cells might be candidates for clinical tumor immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Peng L, Shu SKrauss JC . Treatment of subcutaneous tumor with adoptively transferred T cells. Cell Immunol 1997; 178: 24–32.
Rosenberg SA, Spiess P, Lafreniere R . A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986; 233: 1318–1321.
Plautz GE, Bukowski RM, Novick AC, Klein EA, Kursh ED, Olencki TE et al T-cell adoptive immunotherapy of metastatic renal cell carcinoma. Urology 1999; 54: 617–623; discussion 623–614.
Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL et al Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother 2001; 24: 363–373.
Dudley ME, Rosenberg SA . Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer 2003; 3: 666–675.
Clay TM, Custer MC, Sachs J, Hwu P, Rosenberg SA, Nishimura MI . Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 1999; 163: 507–513.
Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol 2003; 171: 3287–3295.
Hughes MS, Yu YY, Dudley ME, Zheng Z, Robbins PF, Li Y et al Transfer of a TCR gene derived from a patient with a marked antitumor response conveys highly active T-cell effector functions. Hum Gene Ther 2005; 16: 457–472.
Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol 2001; 2: 962–970.
Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006; 314: 126–129.
Kessels HW, Wolkers MC, Schumacher TN . Adoptive transfer of T-cell immunity. Trends Immunol 2002; 23: 264–269.
Hayday AC . Gammadelta cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol 2000; 18: 975–1026.
Kabelitz D, Wesch D, He W . Perspectives of gammadelta T cells in tumor immunology. Cancer Res 2007; 67: 5–8.
Scotet E, Martinez LO, Grant E, Barbaras R, Jeno P, Guiraud M et al Tumor recognition following Vgamma9Vdelta2 T cell receptor interactions with a surface F1-ATPase-related structure and apolipoprotein A-I. Immunity 2005; 22: 71–80.
Chen H, He X, Wang Z, Wu D, Zhang H, Xu C et al. Identification of human T cell receptor gammadelta-recognized epitopes/proteins via CDR3delta peptide-based immunobiochemical strategy. J Biol Chem 2008; 283: 12528–12537.
Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y et al. Adoptive immunotherapy of lung cancer with immobilized anti-TCRgammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther 2009; 8: 1540–1549.
Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood 2003; 102: 200–206.
Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G et al. Targeting human gammadelta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res 2007; 67: 7450–7457.
Wang Z, Zhang T, Hu H, Zhang H, Yang Z, Cui L et al. Targeting solid tumors via T cell receptor complementarity-determining region 3delta in an engineered antibody. Cancer Lett 2008; 272: 242–252.
Yuasa T, Sato K, Ashihara E, Takeuchi M, Maita S, Tsuchiya N et al Intravesical administration of gammadelta T cells successfully prevents the growth of bladder cancer in the murine model. Cancer Immunol Immunother 2009; 58: 493–502.
van der Veken LT, Hagedoorn RS, van Loenen MM, Willemze R, Falkenburg JH, Heemskerk MH . Alphabeta T-cell receptor engineered gammadelta T cells mediate effective antileukemic reactivity. Cancer Res 2006; 66: 3331–3337.
van der Veken LT, Coccoris M, Swart E, Falkenburg JH, Schumacher TN, Heemskerk MH . Alpha beta T cell receptor transfer to gamma delta T cells generates functional effector cells without mixed TCR dimers in vivo. J Immunol 2009; 182: 164–170.
Rock EP, Sibbald PR, Davis MM, Chien YH . CDR3 length in antigen-specific immune receptors. J Exp Med 1994; 179: 323–328.
Adams EJ, Strop P, Shin S, Chien YH, Garcia KC . An autonomous CDR3delta is sufficient for recognition of the nonclassical MHC class I molecules T10 and T22 by gammadelta T cells. Nat Immunol 2008; 9: 777–784.
Xu C, Zhang H, Hu H, He H, Wang Z, Xu Y, et al. Gammadelta T cells recognize tumor cells via CDR3delta region. Mol Immunol 2007; 44: 302–310.
Xi X, Guo Y, Chen H, Xu C, Zhang H, Hu H et al. Antigen specificity of gammadelta T cells depends primarily on the flanking sequences of CDR3delta. J Biol Chem 2009; 284: 27449–27455.
Bunnell BA, Muul LM, Donahue RE, Blaese RM, Morgan RA . High-efficiency retroviral-mediated gene transfer into human and nonhuman primate peripheral blood lymphocytes. Proc Natl Acad Sci USA 1995; 92: 7739–7743.
Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E et al. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol 2005; 175: 5481–5488.
Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T et al. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother 2007; 56: 469–476.
Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J et al. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer 2005; 116: 94–99.
He X, Chen H, Wu D, Cui L, He W . Tandem-epitope peptide: a novel stimulator for gammadeltaT cells in tumor immunotherapy. Cancer Lett; 288: 86–93.
Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P et al Antigen recognition determinants of gammadelta T cell receptors. Science 2005; 308: 252–255.
Dalton JE, Howell G, Pearson J, Scott P, Carding SR . Fas–Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol 2004; 173: 3660–3667.
Nakata M, Smyth MJ, Norihisa Y, Kawasaki A, Shinkai Y, Okumura K et al. Constitutive expression of pore-forming protein in peripheral blood gamma/delta T cells: implication for their cytotoxic role in vivo. J Exp Med 1990; 172: 1877–1880.
Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Krensky AM, Bonneville M et al. Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 2001; 184: 1082–1085.
Christmas SE, Meager A . Production of interferon-gamma and tumour necrosis factor-alpha by human T-cell clones expressing different forms of the gamma delta receptor. Immunology 1990; 71: 486–492.
Poehlein CH, Hu HM, Yamada J, Assmann I, Alvord WG, Urba WJ et al. TNF plays an essential role in tumor regression after adoptive transfer of perforin/IFN-gamma double knockout effector T cells. J Immunol 2003; 170: 2004–2013.
Acknowledgements
This work is supported by two grants, no. 2006AA02Z480 and no. 2007AA021109, from the National Program for Biomedical Hightech, the Ministry of Science and Technology, China. The authors gratefully acknowledged Dr Keng Shen for providing tumor cell line SKOV3 and HO8910.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Xi, X., Cui, L. et al. CDR3δ -grafted γ9δ2T cells mediate effective antitumor reactivity. Cell Mol Immunol 9, 147–154 (2012). https://doi.org/10.1038/cmi.2011.28
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2011.28
Keywords
This article is cited by
-
Hyperactivation and in situ recruitment of inflammatory Vδ2 T cells contributes to disease pathogenesis in systemic lupus erythematosus
Scientific Reports (2015)
-
The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy
Cellular & Molecular Immunology (2015)
-
T-cell-associated cellular immunotherapy for lung cancer
Journal of Cancer Research and Clinical Oncology (2015)
-
Human Vγ9Vδ2-T cells efficiently kill influenza virus-infected lung alveolar epithelial cells
Cellular & Molecular Immunology (2013)
-
Vγ9Vδ2-T lymphocytes have impaired antiviral function in small-for-gestational-age and preterm neonates
Cellular & Molecular Immunology (2013)