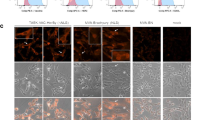
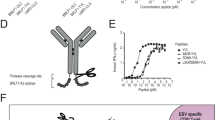
Abstract
Trastuzumab (Herceptin) is a recombinant humanized monoclonal antibody (mAb) directed against an extracellular region of the human epidermal growth-factor receptor type 2 (HER2) protein. We hypothesized that a single adeno-associated virus (AAV)-mediated genetic delivery of an anti-HER2 antibody should be effective in mediating long-term production of anti-HER2 and in suppressing the growth of human tumors in a xenograft model in nude mice. The adeno-associated virus gene transfer vector AAVrh.10αHER2 was constructed based on a non-human primate AAV serotype rh.10 to express the complementary DNAs for the heavy and light chains of mAb 4D5, the murine precursor to trastuzumab. The data show that genetically transferred anti-HER2 selectively bound human HER2 protein and suppressed the proliferation of HER2+ tumor cell lines. A single administration of AAVrh.10αHER2 provided long-term therapeutic levels of anti-HER2 antibody expression without inducing an anti-idiotype response, suppressed the growth of HER2+ tumors and increased the survival of tumor bearing mice. In the context that trastuzumab therapy requires frequent and repeated administration, this strategy might be developed as an alternate platform for delivery of anti-HER2 therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 1984; 312: 513–516.
Fendly BM, Winget M, Hudziak RM, Lipari MT, Napier MA, Ullrich A . Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res 1990; 50: 1550–1558.
Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL . Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182.
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712.
Chan T, Sami A, El-Gayed A, Guo X, Xiang J . HER-2/neu-gene engineered dendritic cell vaccine stimulates stronger HER-2/neu-specific immune responses compared to DNA vaccination. Gene Ther 2006; 13: 1391–1402.
Drebin JA, Link VC, Greene MI . Monoclonal antibodies specific for the neu oncogene product directly mediate anti-tumor effects in vivo. Oncogene 1988; 2: 387–394.
Garrett JT, Rawale S, Allen SD, Phillips G, Forni G, Morris JC et al. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER-2/neu. J Immunol 2007; 178: 7120–7131.
Nahta R Esteva FJ . Trastuzumab: triumphs and tribulations. Oncogene 2007; 26: 3637–3643.
Leyland-Jones B, Arnold A, Gelmon K, Verma S, Ayoub JP, Seidman A et al. Pharmacologic insights into the future of trastuzumab. Ann Oncol 2001; 12 (Suppl 1): S43–S47.
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA 1992; 89: 4285–4289.
De BP, Heguy A, Hackett NR, Ferris B, Leopold PL, Lee J et al. High levels of persistent expression of alpha1-antitrypsin mediated by the nonhuman primate serotype rh.10 adeno-associated virus despite preexisting immunity to common human adeno-associated viruses. Mol Ther 2006; 13: 67–76.
Fang J, Qian JJ, Yi S, Harding TC, Tu GH, VanRoey M et al. Stable antibody expression at therapeutic levels using the 2A peptide. Nat Biotechnol 2005; 23: 584–590.
De BP, Hackett NR, Crystal RG, Boyer JL . Rapid/sustained anti-anthrax passive immunity mediated by co-administration of Ad/AAV. Mol Ther 2008; 16: 203–209.
Limberis MP, Vandenberghe LH, Zhang L, Pickles RJ, Wilson JM . Transduction efficiencies of novel AAV vectors in mouse airway epithelium in vivo and human ciliated airway epithelium in vitro. Mol Ther 2009; 17: 294–301.
Song Y, Lou H, Boyer JL, Limberis MP, Vandenberghe LH, Hackett NR et al. Functional CFTR expression in cystic fibrosis airway epithelial cells by AAV6.2-mediated segmental trans-splicing. Hum Gene Ther 2009; 20: 267–281.
Bunn Jr PA, Helfrich B, Soriano AF, Franklin WA, Varella-Garcia M, Hirsch FR et al. Expression of Her-2/neu in human lung cancer cell lines by immunohistochemistry and fluorescence in situ hybridization and its relationship to in vitro cytotoxicity by trastuzumab and chemotherapeutic agents. Clin Cancer Res 2001; 7: 3239–3250.
Pegram M, Hsu S, Lewis G, Pietras R, Beryt M, Sliwkowski M et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999; 18: 2241–2251.
Spiridon CI, Ghetie MA, Uhr J, Marches R, Li JL, Shen GL et al. Targeting multiple Her-2 epitopes with monoclonal antibodies results in improved antigrowth activity of a human breast cancer cell line in vitro and in vivo. Clin Cancer Res 2002; 8: 1720–1730.
Jia LT, Zhang LH, Yu CJ, Zhao J, Xu YM, Gui JH et al. Specific tumoricidal activity of a secreted proapoptotic protein consisting of HER2 antibody and constitutively active caspase-3. Cancer Res 2003; 63: 3257–3262.
Kern JA, Torney L, Weiner D, Gazdar A, Shepard HM, Fendly B . Inhibition of human lung cancer cell line growth by an anti-p185HER2 antibody. Am J Respir Cell Mol Biol 1993; 9: 448–454.
Martinez-Ramirez A, Rodriguez-Perales S, Melendez B, Martinez-Delgado B, Urioste M, Cigudosa JC et al. Characterization of the A673 cell line (Ewing tumor) by molecular cytogenetic techniques. Cancer Genet Cytogenet 2003; 141: 138–142.
Dubel S . Recombinant therapeutic antibodies. Appl Microbiol Biotechnol 2007; 74: 723–729.
Harries M Smith I . The development and clinical use of trastuzumab (Herceptin). Endocr Relat Cancer 2002; 9: 75–85.
Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J . Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res 1998; 58: 2825–2831.
Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ . Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1998; 17: 2235–2249.
Valabrega G, Montemurro F, Aglietta M . Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol 2007; 18: 977–984.
Bakker JM, Bleeker WK, Parren PW . Therapeutic antibody gene transfer: an active approach to passive immunity. Mol Ther 2004; 10: 411–416.
Marasco WA . Therapeutic antibody gene transfer. Nat Biotechnol 2005; 23: 551–552.
Liu XY, Pop LM, Vitetta ES . Engineering therapeutic monoclonal antibodies. Immunol Rev 2008; 222: 9–27.
Li J, Menzel C, Meier D, Zhang C, Dubel S, Jostock T . A comparative study of different vector designs for the mammalian expression of recombinant IgG antibodies. J Immunol Methods 2007; 318: 113–124.
Hotta A, Kamihira M, Itoh K, Morshed M, Kawabe Y, Ono K et al. Production of anti-CD2 chimeric antibody by recombinant animal cells. J Biosci Bioeng 2004; 98: 298–303.
Noel D, Pelegrin M, Kramer S, Jacquet C, Skander N, Piechaczyk M . High in vivo production of a model monoclonal antibody on adenoviral gene transfer. Hum Gene Ther 2002; 13: 1483–1493.
Watanabe M, Boyer JL, Hackett NR, Qiu J, Crystal RG . Genetic delivery of the murine equivalent of bevacizumab (avastin), an anti-vascular endothelial growth factor monoclonal antibody, to suppress growth of human tumors in immunodeficient mice. Hum Gene Ther 2008; 19: 300–310.
Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T . IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 2000; 1: 376–382.
Noel D, Pelegrin M, Marin M, Biard-Piechaczyk M, Ourlin JC, Mani JC et al. In vitro and in vivo secretion of cloned antibodies by genetically modified myogenic cells. Hum Gene Ther 1997; 8: 1219–1229.
Tjelle TE, Corthay A, Lunde E, Sandlie I, Michaelsen TE, Mathiesen I et al. Monoclonal antibodies produced by muscle after plasmid injection and electroporation. Mol Ther 2004; 9: 328–336.
Chen J, Su C, Lu Q, Shi W, Zhang Q, Wang X et al. Generation of adenovirus-mediated anti-CD20 antibody and its effect on B-cell deletion in mice and nonhuman primate cynomolgus monkey. Mol Cancer Ther 2008; 7: 1562–1568.
Ho DT, Wykoff-Clary S, Gross CS, Schneider D, Jin F, Kretschmer PJ et al. Growth inhibition of an established A431 xenograft tumor by a full-length anti-EGFR antibody following gene delivery by AAV. Cancer Gene Ther 2009; 16: 184–194.
Deshane J, Loechel F, Conry RM, Siegal GP, King CR, Curiel DT . Intracellular single-chain antibody directed against erbB2 down-regulates cell surface erbB2 and exhibits a selective anti-proliferative effect in erbB2 overexpressing cancer cell lines. Gene Ther 1994; 1: 332–337.
Deshane J, Siegal GP, Alvarez RD, Wang MH, Feng M, Cabrera G et al. Targeted tumor killing via an intracellular antibody against erbB-2. J Clin Invest 1995; 96: 2980–2989.
Xu YM, Wang LF, Jia LT, Qiu XC, Zhao J, Yu CJ et al. A caspase-6 and anti-human epidermal growth factor receptor-2 (HER2) antibody chimeric molecule suppresses the growth of HER2-overexpressing tumors. J Immunol 2004; 173: 61–67.
Jiang M, Shi W, Zhang Q, Wang X, Guo M, Cui Z et al. Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin Cancer Res 2006; 12: 6179–6185.
Nguyen AT, Dow AC, Kupiec-Weglinski J, Busuttil RW, Lipshutz GS . Evaluation of gene promoters for liver expression by hydrodynamic gene transfer. J Surg Res 2008; 148: 60–66.
Loeb JE, Cordier WS, Harris ME, Weitzman MD, Hope TJ . Enhanced expression of transgenes from adeno-associated virus vectors with the woodchuck hepatitis virus posttranscriptional regulatory element: implications for gene therapy. Hum Gene Ther 1999; 10: 2295–2305.
Hackett NR, Kaminsky SM, Sondhi D, Crystal RG . Antivector and antitransgene host responses in gene therapy. Curr Opin Mol Ther 2000; 2: 376–382.
Pelegrin M, Marin M, Noel D, Piechaczyk M . Genetically engineered antibodies in gene transfer and gene therapy. Hum Gene Ther 1998; 9: 2165–2175.
Cesco-Gaspere M, Zentilin L, Giacca M, Burrone OR . Boosting anti-idiotype immune response with recombinant AAV enhances tumour protection induced by gene gun vaccination. Scand J Immunol 2008; 68: 58–66.
Acknowledgements
We thank B Ferris, BP De and A Bajak for technical assistance, and T Bryan and N Mohamed for their help in preparing this paper. These studies were supported, in part, by U01 HL66952 and the Will Rogers Memorial Fund, Los Angeles, CA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
These studies were supported, in part, by U01 HL66952.
Rights and permissions
About this article
Cite this article
Wang, G., Qiu, J., Wang, R. et al. Persistent expression of biologically active anti-HER2 antibody by AAVrh.10-mediated gene transfer. Cancer Gene Ther 17, 559–570 (2010). https://doi.org/10.1038/cgt.2010.11
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2010.11
Keywords
This article is cited by
-
Anti-HER2 antibody therapy using gene-transduced adipocytes for HER2-positive breast cancer
Breast Cancer Research and Treatment (2020)
-
Gene therapy using plasmid DNA-encoded anti-HER2 antibody for cancers that overexpress HER2
Cancer Gene Therapy (2016)
-
Recombinant AAV-mediated in vivo long-term expression and antitumour activity of an anti-ganglioside GM3(Neu5Gc) antibody
Gene Therapy (2015)
-
Prevention and reversal of severe mitochondrial cardiomyopathy by gene therapy in a mouse model of Friedreich's ataxia
Nature Medicine (2014)
-
Gene-based passive antibody protection from HIV
Nature Biotechnology (2012)