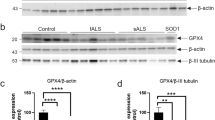
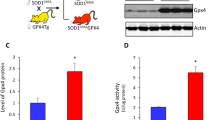
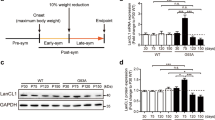
Abstract
The mechanism by which Cu2+/Zn2+ superoxide dismutase (SOD1) mutants lead to motor neuron degeneration in familial amyotrophic lateral sclerosis (FALS) is unknown. We show that oxidative reactions triggered by hydrogen peroxide and catalyzed by A4V and I113T mutant but not wild-type SOD1 inactivated the glutamate transporter human GLT1. Chelation of the copper ion of the prosthetic group of A4V prevented GLT1 inhibition. GLT1 was a selective target of oxidation mediated by SOD1 mutants, and its reactivity was confined to the intracellular carboxyl-terminal domain. The antioxidant Mn(III)TBAP rescued GLT1 from inhibition. Because inactivation of GLT1 results in neuronal degeneration, we propose that toxic properties of SOD1 mutants lead to neuronal death via an excitotoxic mechanism in SOD1-linked FALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rosen, D. R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Gurney, M. E. et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264, 1772–1775 (1994).
Bruijn, L. I. et al. Elevated free nitrotyrosine levels, but not protein-bound nitrotyrosine or hydroxyl radicals, throughout amyotrophic lateral sclerosis (ALS)-like disease implicate tyrosine nitration as an aberrant in vivo property of one familial ALS-linked superoxide dismutase 1 mutant. Proc. Natl. Acad. Sci. USA 94, 7606– 7611 (1997).
Bogdanov, M. B., Ramos, L. E., Xu, Z. & Beal, M. F. Elevated hydroxyl radical generation in vivo in an animal model of amyotrophic lateral sclerosis. J. Neurochem. 71, 1321– 1324 (1998).
Ferrante, R. J. et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J. Neurochem. 69, 2064–2074 (1997).
Borchelt, D. R. et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc. Natl. Acad. Sci. USA 91, 8292–8296 ( 1994).
Borchelt, D. R. et al. Superoxide dismutase 1 subunits with mutations linked to familial amyotrophic lateral sclerosis do not affect wild-type subunit function. J. Biol. Chem. 270, 3234–3238 (1995).
Deng, H-X. et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science 261, 1047– 1051 (1993).
Yim, H-S., Kang, J-H., Chock, P. B., Stadtman, E. R. & Yim, M. B. A familial amyotrophic lateral sclerosis-associated A4V Cu, Zn superoxide dismutase mutant has a lower Km for hydrogen peroxide. Correlation between clinical severity and the Km value. J. Biol. Chem. 272, 8861– 8863 (1997).
Wiedau-Pazos, M. et al. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science 271, 515– 518 (1996).
Yim, M. B. et al. A gain of function of an amyotrophic lateral sclerosis-associated Cu, Zn superoxide dismutase mutant: an enhancement of free radical formation due to a decrease in Km for hydrogen peroxide. Proc. Natl. Acad. Sci. USA 93, 5709–5714 (1996).
Rothstein, J. D. et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16, 675–686 ( 1996).
Haugeto, O. et al. Brain glutamate transporter proteins form homomultimers. J. Biol. Chem. 271, 27715–27722 (1996).
Lehre, K. P. & Danbolt, N. C. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J. Neurosci. 18, 8751–8757 (1998).
Rothstein, J. D., Martin, L. J. & Kuncl, R. W. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N. Engl. J. Med. 326, 1464–1468 (1992).
Rothstein, J. D., Van Kammen, M., Levey, A. I., Martin, L. J. & Kuncl, R. W. Selective loss of glial glutamate transporter GLT1 in amyotrophic lateral sclerosis. Ann. Neurol. 38, 73–84 ( 1995).
Bristol, L. A. & Rothstein, J. D. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann. Neurol. 39, 676–679 (1996).
Aoki, M. et al. Mutations in the glutamate transporter EAAT2 gene do not cause abnormal EAAT2 transcripts in amyotrophic lateral sclerosis. Ann. Neurol. 43, 645–653 ( 1998).
Lin, C-L. G. et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602 (1998).
Canton, T., Pratt, J., Stutzmann, J-M., Imperato, A. & Boireau, A. Glutamate uptake is decreased tardively in the spinal cord of FALS mice. Neuroreport 9, 775–778 (1998).
Bruijin, L. I. et al. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron 18, 327–338 (1997).
Pedersen, W. A. et al. Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann. Neurol. 44, 819–824 (1998).
Trotti, D. et al. Peroxynitrite inhibits glutamate transporter subtypes. J. Biol. Chem. 271, 5976–5979 (1996).
Trotti, D., Nussberger, S., Volterra, A. & Hediger, M. A. Differential modulation of the uptake currents by redox interconversion of cysteine residues in the human neuronal glutamate transporter EAAC1. Eur. J. Neurosci. 9, 2207–2212 (1997).
Trotti, D., Danbolt, N. C. & Volterra, A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol. Sci. 19, 328–334 (1998).
Taylor, P. M., Kaur, S., Mackenzie, B. & Peter, G. J. Amino-acid-dependent modulation of amino acid transporter in Xenopus laevis oocytes. J. Exp. Biol. 199, 923–931 (1996).
Robberecht, W. et al. Cu/Zn superoxide dismutase activity in familial and sporadic amyotrophic lateral sclerosis. J. Neurochem. 62, 384–387 (1994).
Tu, P-H., Gurney, M. E., Julien, J-P., Lee, V. M. & Trojanowski, J. Q. Oxidative stress, mutant SOD1, and neurofilament pathology in transgenic mouse models of human motor neuron disease. Lab. Invest. 76, 441– 456 (1997).
Siddique, T. & Deng, H. X. Genetics of amyotrophic lateral sclerosis. Hum. Mol. Gen. 5, 1465– 1470 (1996).
Gillis, K. D. in Single Channel Recording 2nd edn. (eds. Sakmann, B. & Neher, E.) 155–198 (Plenum, New York, 1995).
Davies, K. J., Delsignore, M. E. & Lin, S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J. Biol. Chem. 262 , 9902–9907 (1987).
Zerangue, N. & Kavanaugh, M. P. Flux coupling in a neuronal glutamate transporter. Nature 383, 634– 637 (1996).
Davies, K. J. Protein damage and degeneration by oxygen radicals. I. General aspects. J. Biol. Chem. 262, 9895–9901 (1987).
Grunewald, M., Bendahan, A. & Kanner, B. I. Biotinylation of single cysteine mutants of the glutamate transporter GLT1 from rat brain reveals its unusual topology. Neuron 21, 623–632 ( 1998).
Day, B. J., Fridovich, I. & Crapo, J. D. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch. Biochem. Biophys. 347, 256–262 (1997).
Batinic-Haberle, I., Liochev, S. I., Spasojevic, I. & Fridovich, I. A potent superoxide dismutase mimic: Manganese β-octabromo-meso-etrakis-(N-methylpyridinium-4-yl) porphyrin. Arch. Biochem. Biophys. 343, 225–233 (1997).
Mu, X., He, J., Anderson, D. W., Trojanowski, J. Q. & Springer, J. E. Altered expression of bcl-2 and bax mRNA in amyotrophic lateral sclerosis spinal cord motor neurons. Ann. Neurol. 40, 379–386 (1996).
Eisen, A. Amyotrophic lateral sclerosis is a multifactorial disease. Muscle Nerve 18, 741–752 ( 1995).
Brown, R. H. Jr. Free radicals, programmed cell death and muscular dystrophy. Curr. Opin. Neurol. 8, 373– 378 (1995).
Brown, R. H. Jr. Amyotrophic lateral sclerosis: recent insights from genetics and transgenic mice. Cell 80, 687– 692 (1995).
van Iwaarden, P. R., Driessen, A. J. & Koning, W. N. What we can learn from the effects of thiol reagents on transport proteins. Biochem. Biophys. Acta 1113, 161–170 (1992).
Davies, K. J. A. & Delsignore, M. E. Protein damage and degradation by oxygen radicals. III. Modification of secondary and tertiary structure. J. Biol. Chem. 262, 9908–9913 (1987).
Davies, K. J. A., Lin, S. W. & Pacifici, R. E. Protein damage and degradation by oxygen radicals. IV. Degradation of denatured protein. J. Biol. Chem. 262, 9914–9920 (1987).
Tanaka, K. et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT1. Science 276, 1699–1702 (1997).
Bensimon, G., Lacomblez, L. & Meininger, V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/riluzole study group. N. Engl. J. Med. 330, 585–591 (1994).
Lacomblez, L., Bensimon, G., Leigh, P. N., Guillet, P. & Meininger, V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis/riluzole study group II. Lancet 347, 1425– 1431 (1996).
Gurney, M. E., Fleck, T. J., Himes, C. S. & Hall, E. D. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology 50, 62– 66 (1998).
Arriza, J. L. et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 14, 5559–5569 (1994).
Acknowledgements
We thank C. Shayakul for advice in the cloning of human GLT1, P. Fong for providing the vector pTLNII for oocyte expression and P. Pasinelli and Y. Segal for comments on the manuscript. This work was supported by the Paralyzed Veterans of America-SCRF and Telethon-Italy to D.T., the Swiss National Foundation to A.R., a grant from NINDS (NS32001) to M.A.H., ALS Association and Muscular Dystrophy Association to M.A.H. and R.H.B. and the Norwegian Research Council to N.C.D. R.H.B. was also supported by the Pierre L. de Bourgknecht ALS Research Foundation and grants from NINDS (1PO1NS31248-05 and 5F32HS10064) and NIA (1PO1Ag12992-04).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Trotti, D., Rolfs, A., Danbolt, N. et al. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci 2, 427–433 (1999). https://doi.org/10.1038/8091
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/8091
This article is cited by
-
In Vitro Models of Amyotrophic Lateral Sclerosis
Cellular and Molecular Neurobiology (2023)
-
Improving clinical trial outcomes in amyotrophic lateral sclerosis
Nature Reviews Neurology (2021)
-
Astragaloside IV and echinacoside benefit neuronal properties via direct effects and through upregulation of SOD1 astrocyte function in vitro
Naunyn-Schmiedeberg's Archives of Pharmacology (2021)
-
Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases
Translational Neurodegeneration (2017)
-
Ultrastructural features of aberrant glial cells isolated from the spinal cord of paralytic rats expressing the amyotrophic lateral sclerosis-linked SOD1G93A mutation
Cell and Tissue Research (2017)