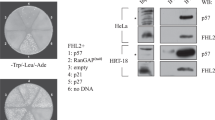
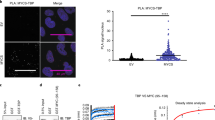
Abstract
The adenovirus E1A and SV40 large-T-antigen oncoproteins bind to members of the p300/CBP transcriptional coactivator family. Binding of p300/CBP is implicated in the transforming mechanisms of E1A and T-antigen oncoproteins. A common region of the T antigen is critical for binding both p300/CBP and the tumour suppressor p53 (ref. 1), suggesting a link between the functions of p53 and p300. Here we report that p300/CBP binds to p53 in the absence of viral oncoproteins, and that p300 and p53 colocalize within the nucleus and coexist in a stable DNA-binding complex. Consistent with its ability to bind to p300, E1A disrupted functions mediated by p53. It reduced p53-mediated activation of the p21 and bax promoters, and suppressed p53-induced cell-cycle arrest and apoptosis. We conclude that members of the p300/CBP family are transcriptional adaptors for p53, modulating its checkpoint function in the G1 phase of the cell cycle and its induction of apoptosis. Disruption of p300/p53-dependent growth control may be part of the mechanism by which E1A induces cell transformation. These results help to explain how p53 mediates growth and checkpoint control, and how members of the p300/CBP family affect progression from G1 to the S phase of the cell cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lill, N. L. et al. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J. Virol. 71, 129–137 (1997).
Eckner, R. et al. Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol. 16, 3454–3464 (1996).
Chowdary, D. R. et al. Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol. Cell. Biol. 14, 1997–2003 (1994).
Steegenga, W. T. et al. Adenovirus E1A proteins inhibit activation of transcription by p53. Mol. Cell. Biol. 16, 2101–2109 (1996).
Eckner, R. et al. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8, 869–884 (1994).
Kern, S. E. et al. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science 256, 827–830 (1992).
Stein, R. W. et al. Analysis of E1A mediated growth regulation functions binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J. Virol. 64, 4421–4427 (1990).
Howe, J. A. & Bayley, S. T. Effects of Ad5 E1A mutant viruses on the cell cycle in relation to the binding of cellular proteins including the retinoblastoma protein and cyclin A. Virology 186, 15–24 (1992).
El-Deiry, W. S. et al. WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 (1993).
Miyashita, T. & Reed, J. C. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell 80, 293–299 (1995).
Yang, X. J. et al. Ap300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382, 319–324 (1996).
Eckner, R. et al. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 10, 2478–2490 (1996).
Yao, T.-P. et al. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc. Natl Acad. Sci. USA 93, 10626–10631 (1996).
Diller, L. et al. p53 functions as a cell cycle control protein in osteosarcomas. Mol. Cell. Biol. 10, 5772–5781 (1990).
Haupt, Y. et al. Induction of apoptosis in HeLa cells by transactivation-deficient p53. Genes Dev. 9, 2170–2183 (1995).
Chen, X. et al. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10, 2438–2451 (1996).
Bayley, S. T. & Mymryk, J. S. Adenovirus E1A proteins and transformation. Int. J. Oncol. 5, 425–444 (1994).
Caporossi, D. & Bacchetti, S. Definition of the adenovirus type 5 functions involved in the induction of chromosomal aberrations in human cells. J. Gen. Virol. 71, 801–808 (1990).
Abraham, S. E. et al. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene 8, 1639–1647 (1993).
Ko, L. J. & Prives, C. p53: puzzle and paradigm. Genes Dev. 10, 1054–1072 (1996).
Baker, S. J. et al. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science 249s, 912–915 (1990).
White, E. et al. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J. Virol. 65, 2968–2978 (1991).
Kahana, J. & Silver, P. in Current Protocols in Molecular Biology(eds Ausubel, F. M. et al.) 9.7.22–9.7.28 (Greene and Wiley Interscience, New York, (1996)).
Zhu, L. et al. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 7, 1111–1125 (1993).
Ausubel, F. M. et al. Current Protocols in Molecular Biology(Greene and Wiley Interscience, New York, (1996)).
Krek, W., Livingston, D. M. & Shirodkar, S. Binding to DNA and the retinoblastoma gene product promoted by complex formation of different E2F family members. Science 262, 1557–1560 (1993).
Chittenden, T., Livingston, D. M. & DeCaprio, J. A. Cell cycle analysis of E2F in primary human T cells reveals novel E2F complexes and biochemically distinct forms of free E2F. Mol. Cell. Biol. 13, 3975–3983 (1993).
Friedlander, et al. Amutant p53 that discriminates between p53-responsive genes cannot induce apoptosis. Mol. Cell. Biol. 16, 4961–4971 (1996).
Qin, X.-Q. et al. The transcription factor E2F-1 is a downstream target of RB action. Mol. Cell. Biol. 15, 742–755 (1995).
Somasundaram, K. & El-Deiry, W. S. Inhibition of p53-mediated transactivation and cell cycle arrest by E1A through its p300/CBP-interacting region. Oncogene 14, 1047–1057 (1997).
Acknowledgements
We thank everyone who provided reagents used in this study; J. Kahana, P. Silver, C. Jost and W. G. Kaelin Jr for providing unpublished reagents; P. Adams, R. Eckner, M. Ewen, O. Iliopoulos, R. Scully, T.-P. Yao and members of the Division of Neoplastic Disease Mechanisms for discussions; M.Modabber for graphics; and M. Simone for flow cytometry. This work was supported by grants from the American Cancer Society (N.L.L.), the Howard Hughes Medical Institute (S.R.G.), The Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation (D.G.), the Dana-Farber/Sandoz Drug Discovery Program, and the National Cancer Institute (J. DeC. and D.M.L.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lill, N., Grossman, S., Ginsberg, D. et al. Binding and modulation of p53 by p300/CBP coactivators. Nature 387, 823–827 (1997). https://doi.org/10.1038/42981
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/42981
This article is cited by
-
FIT links c-Myc and P53 acetylation by recruiting RBBP7 during colorectal carcinogenesis
Cancer Gene Therapy (2023)
-
Readout of histone methylation by Trim24 locally restricts chromatin opening by p53
Nature Structural & Molecular Biology (2023)
-
Deciphering the acetylation code of p53 in transcription regulation and tumor suppression
Oncogene (2022)
-
p53 signaling in cancer progression and therapy
Cancer Cell International (2021)
-
RETRACTED ARTICLE: Molecular mechanisms in progression of HPV-associated cervical carcinogenesis
Journal of Biomedical Science (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.