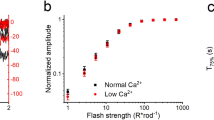
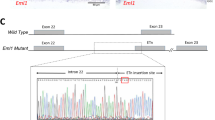
Abstract
Arrestins are soluble cytoplasmic proteins that bind to G-protein-coupled receptors, thus switching off activation of the G protein and terminating the signalling pathway that triggers the cellular response1,2. Although visual arrestin has been shown to quench the catalytic activity of photoexcited, phosphorylated rhodopsin in a reconstituted system3, its role in the intact rod cell remains unclear because phosphorylation alone reduces the catalytic activity of rhodopsin4,5,6. Here we have recorded photocurrents of rods from transgenic mice in which one or both copies of the arrestin gene were disrupted. Photoresponses were unaffected when arrestin expression was halved, indicating that arrestin binding is not rate limiting for recovery of the rod photoresponse, as it is in Drosophila7,8. With arrestin absent, the flash response displayed a rapid partial recovery followed by a prolonged final phase. This behaviour indicates that an arrestin-independent mechanism initiates the quench of rhodopsin's catalytic activity and that arrestin completes the quench. The intensity dependence of the photoresponse in rods lacking arrestin further suggests that, although arrestin is required for normal signal termination, it does not participate directly in light adaptation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
References
Wilson, C. J. & Applebury, M. L. Arresting G-protein coupled receptor activity. Curr. Biol. 3, 683–686 (1993).
Palczewski, K. Structure and functions of arrestins. Protein Sci. 3, 1355–1361 (1994).
Wilden, U., Hall, S. W. & Kuhn, H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc. Natl Acad. Sci. USA 83, 1174–1178 (1986).
Miller, J. L., Fox, D. A. & Litman, B. J. Amplification of phosphodiesterase activation is greatly reduced by rhodopsin phosphorylation. Biochemistry 25, 4983–4988 (1986).
Arshavsky, V. Y., Antoch, M. P., Dizhoor, A. M., Rakhilin, S. V. & Philippov, P. P. in Retinal Proteins (ed. Ovchinnikov, Y. A.) 497–503 (VNU, Utrecht, (1987)).
Wilden, U. Duration and amplitude of the light-induced cGMP hydrolysis in vertebrate photoreceptors are regulated by multiple phosphorylation of rhodopsin and by arrestin binding. Biochemistry 34, 1446–1454 (1995).
Dolph, P. J. et al. Arrestin function in inactivation of G protein-coupled receptor rhodopsin in vivo. Science 260, 1910–1916 (1993).
Ranganathan, R. & Stevens, C. F. Arrestin binding determines the rate of inactivation of the G protein-coupled receptor rhodopsin in vivo. Cell 81, 841–848 (1995).
Palczewski, K. & Smith, W. C. Splice variants of arrestins. Exp. Eye Res. 63, 599–602 (1996).
Penn, J. S. & Williams, T. P. Photostasis: regulation of daily photon-catch by rat retinas in response to various cyclic illuminances. Exp. Eye Res. 43, 915–928 (1986).
Schremser, J.-L. & Williams, T. P. Rod outer segment (ROS) renewal as a mechanism for adaptation to a new intensity environment. I. Rhodopsin levels and ROS length. Exp. Eye Res. 61, 17–24 (1995).
Lamb, T. D. & Pugh, E. N. J Aquantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J. Physiol. (Lond.) 449, 719–758 (1992).
Pugh, E. N. J & Lamb, T. D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1141, 111–149 (1993).
Ebrey, T. G. The thermal decay of the intermediates of rhodopsin in situ. Vis. Res. 8, 965–982 (1968).
Cone, R. A. & Cobbs, W. H. Rhodopsin cycle in the living eye of the rat. Nature 221, 820–822 (1969).
Chen, J., Makino, C. L., Peachey, N. S., Baylor, D. A. & Simon, M. I. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science 267, 374–377 (1995).
Palczewski, K., Pulvermiller, A., Buczylko, J., Gutmann, C. & Hofmann, K. P. Binding of inositol phosphates to arrestin. FEBS Lett. 295, 195–199 (1991).
Palczewski, K., Rispoli, G. & Detwiler, P. B. The influence of arrestin (48K protein) and rhodopsin kinase on visual transduction. Neuron 8, 117–126 (1992).
Baylor, D. A. & Hodgkin, A. L. Changes in time scale and sensitivity in turtle photoreceptors. J. Physiol. (Lond.) 242, 729–758 (1974).
Nakatani, K., Tamura, T. & Yau, K.-W. Light adaptation in retinal rods of the rabbit and two other nonprimate mammals. J. Gen. Physiol. 97, 413–435 (1991).
Fuchs, S. et al. Ahomozygous 1-base pair deletion in the arrestin gene is a frequent cause of Oguchi disease in Japanese. Nature Genet. 10, 360–362 (1995).
Ramirez-Solis, R., Davis, A. C. & Bradley, A. in Methods in Enzymology: Guide to Techniques in Mouse Development (eds Wassarman, P. M. & DePamphilis, M. L.) 855–878 (Academic, San Diego, (1993)).
Adamus, G., Machniki, M. & Seigel, G. M. Apoptotic retinal cell death induced by antirecoverin autoantibodies of cancer-associated retinopathy. Invest. Ophthalmol. Vis. Sci. 38, 283–291 (1997).
Molday, R. S. & MacKenzie, D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry 22, 653–660 (1983).
Amatruda, T. T., Gautam, N., Fong, H. K., Northrup, J. K. & Simon, M. I. The 35- and 36-kDa beta subunits of GTP-binding regulatory proteins are products of separate genes. J. Biol. Chem. 263, 5008–5011 (1988).
Arshavsky, V. Y., Dumke, C. L. & Bownds, M. D. Noncatalytic cGMP-binding sites of amphibian rod cGMP phosphodiesterase control interaction with its inhibitory γ-subunits. J. Biol. Chem. 267, 24501–24507 (1992).
Lee, R. H., Lieberman, B. S. & Lolley, R. N. Retinal accumulation of the phosphoducin/Tβγ and transducin complexes in developing normal mice and in mice and dogs with inherited retinal degeneration. Exp. Eye Res. 51, 325–333.
Dizhoor, A. M. et al. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science 251, 915–918 (1991).
Raport, C. J. et al. Downregulation of cyclic GMP phosphodiesterase induced by expression of GTPase-deficient cone transducin in mouse rod photoreceptors. Invest. Ophthalmol. Vis. Sci. 35, 2932–2947 (1994).
Baylor, D. A., Lamb, T. D. & Yau, K.-W. Responses of retinal rods to single photons. J. Physiol. (Lond.) 288, 613–634 (1979).
Acknowledgements
This work was supported by grants from the National Eye Institute (D.A.B.), the National Institute on Aging (M.I.S.), the National Institute of General Medical Sciences (R.L.D.), the Ruth and Milton Steinbach Fund (D.A.B., J.C.), The McKnight Endowment Fund for Neuroscience (D.A.B.), Research to Prevent Blindness (J.C., C.L.M.) and Lions' of Massachusetts (C.L.M.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, J., Dodd, R., Makino, C. et al. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature 389, 505–509 (1997). https://doi.org/10.1038/39068
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/39068
This article is cited by
-
Structural and functional distinctions of co-resident microglia and monocyte-derived macrophages after retinal degeneration
Journal of Neuroinflammation (2022)
-
Retinal Layer Separation (ReLayS) method enables the molecular analysis of photoreceptor segments and cell bodies, as well as the inner retina
Scientific Reports (2022)
-
Tracking distinct microglia subpopulations with photoconvertible Dendra2 in vivo
Journal of Neuroinflammation (2021)
-
Functional identification of an opsin kinase underlying inactivation of the pineal bistable opsin parapinopsin in zebrafish
Zoological Letters (2021)
-
Functional modulation of phosphodiesterase-6 by calcium in mouse rod photoreceptors
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.