Abstract
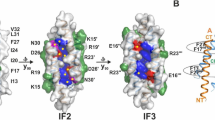
The ion-channel forming colicins A, B, El, Ia, Ib and N all kill bacterial cells selectively by co-opting bacterial active-transport pathways and forming voltage-gated ion conducting channels across the plasma membrane of the target bacterium1,2. The crystal structure of colicin Ia reveals a molecule 210 Å long with three distinct functional domains arranged along a backbone of two extraordinarily long α-helices. A central domain at the bend of the hairpin-like structure mediates specific recognition and binding to an outer-membrane receptor3. A second domain mediates translocation across the outer membrane via the TonB transport pathway4; the TonB-box5 recognition element of colicin Ia is on one side of three 80 Å-long helices arranged as a helical sheet. A third domain is made up of 10 α-helices which form a voltage-activated and voltage-gated ion conducting channel across the plasma membrane of the target cell. The two 160 Å-long α-helices that link the receptor-binding domain to the other domains enable the colicin Ia molecule to span the periplasmic space and contact both the outer and plasma membranes simultaneously during function6,7.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cramer, W. A. et al. Annu. Rev. Biophys. Biomol. Struct. 24, 611–641 (1995).
Stroud, R. Curr. Opin. Struct. Biol. 5, 514–520 (1995).
Nau, C. D. & Konisky, J. J. Bacteriol. 171, 1041–1047 (1989).
Braun, V. FEMS Microbiol. Rev. 16, 295–307 (1995).
Schramm, E., Mende, J., Braun, V. & Kamp, R. M. J. Bacteriol. 169, 3350–3357 (1987).
Benedetti, H., Lloubes, R., Lazdunski, C. & Letellier, L. EMBO J. 11, 441–447 (1992).
Duche, D., Letellier, L., Geli, V., Benedetti, H. & Baty, D. J. Bacteriol. 177, 4935–4939 (1995).
Baty, D. et al. Mol. Microbiol. 2, 807–811 (1988).
Benedetti, H. et al. J. Mol. Biol. 217, 429–439 (1991).
Ghosh, P., Mel, S. F. & Stroud, R. M. Nature Struct. Biol. 1, 597–604 (1994).
Konisky, J. & Richards, F. M. J. Biol. Chem. 245, 2972–2978 (1970).
Brunden, K. R., Cramer, W. A. & Cohen, F. S. J. Biol. Chem. 259, 190–196 (1984).
Mende, J. & Braun, V. Mol. Microbiol. 4, 1523–1533 (1990).
Parker, M. W., Postma, J. P., Pattus, F., Tucker, A. D. & Tsernoglou, D. J. Mol. Biol. 224, 639–657 (1992).
Lakey, J. H., Duche, D., Gonzalez-Manas, J. M., Baty, D. & Pattus, F. J. Mol. Biol. 230, 1055–1067 (1993).
Song, H. Y., Cohen, F. S. & Cramer, W. A. J. Bacteriol. 173, 2927–2934 (1991).
Rutz, J. M. et al. Science 258, 471–475 (1992).
Murphy, C. K., Kalve, V. I. & Klebba, P. E. J. Bacteriol. 172, 2736–2746 (1990).
Jeanteur, D., Pattus, F. & Timmins, P. A. J. Mol. Biol. 235, 898–907 (1994).
Slatin, S. L., Qiu, X. Q., Jakes, K. S. & Finkelstein, A. Nature 371, 158–161 (1994).
Qiu, X. Q., Jakes, K. S., Kienker, P. K., Finkelstein, A. & Slatin, S. L. J. Gen. Physiol. 107, 313–328 (1996).
Leduc, M., Frehel, C. & van Heijenoort, J. J. Bacteriol 161, 627–635 (1985).
Ghosh, P., Mel, S. F. & Stroud, R. M. J. Membr. Biol. 134, 85–92 (1993).
Mel, S. F., Falick, A. M., Burlingame, A. L. & Stroud, R. M. Biochemistry 32, 9473–9479 (1993).
Otwinowski, Z. & Minor, W. in Data Collecting and Processing (eds Sawyer, L., Isaacs, N. & Bailey, S.) 556–562 (SERC Daresbury Laboratory, Warrington, UK, 1993).
Abrahams, J. P. & Leslie, A. G. W. Acta Crystallogr. D 52, 30–42 (1996).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Read, R. J. Acta Crystallogr. A 42, 140–149 (1986).
Brunger, A. T. X-PLOR Version 3.1, a System for Crystallography and NMR (Yale University Press, New Haven, CT, 1992).
Laskowski, R. A., MacArthur, M. W., Morris, A. L. & Thornton, J. M. J. Appl. Crystallogr. 26, 283–291 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiener, M., Freymann, D., Ghosh, P. et al. Crystal structure of colicin Ia. Nature 385, 461–464 (1997). https://doi.org/10.1038/385461a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/385461a0
This article is cited by
-
Story of Pore-Forming Proteins from Deadly Disease-Causing Agents to Modern Applications with Evolutionary Significance
Molecular Biotechnology (2023)
-
The P. aeruginosa effector Tse5 forms membrane pores disrupting the membrane potential of intoxicated bacteria
Communications Biology (2022)
-
Antagonistic functions between the RNA chaperone Hfq and an sRNA regulate sensitivity to the antibiotic colicin
The EMBO Journal (2013)
-
Swimming against the tide: progress and challenges in our understanding of colicin translocation
Nature Reviews Microbiology (2010)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.