Abstract
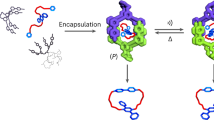
THE interior of cage-like molecules can be considered to provide a new phase of matter1,2, in which it becomes possible to stabilize reactive intermediates3 and to observe new forms of stereoisomerism4. Cage-like molecular complexes that self-assemble through weak intermolecular forces are dynamic species5,6, encapsulating guest molecules reversibly. They can persist over timescales ranging from microseconds to hours, long enough for chemical processes to take place within them. Here we report the acceleration of a Diels–Alder reaction by encapsulation of the reac-tants in a self-assembling molecular capsule7,8. Although product inhibition (lack of dissociation) prevents the system from showing true catalytic behaviour, there is clear evidence for a rate increase of over two orders of magnitude owing to the effective enhancement of concentration inside the capsule.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Sherman, J. C. & Cram, D. J. J. Am. Chem. Soc. 117, 4527–4528 (1989).
Garel, L., Dutasta, J. -P. & Collet, A. Angew. Chem. Int. Edn Engl. 32, 1169–1171 (1993).
Cram, D. J., Tanner, M. E. & Thomas, R. Angew. Chem. Int. Edn Engl. 30, 1024–1027 (1991).
Timmerman, P., Verboom, W., van Veggel, F. C. J. M., van Duynhoven J. P. M. & Reinhoudt, D. N. Angew. Chem. Int. Edn Engl. 33, 2345–2348 (1994).
Wyler, R., de Mendoza, J. & Rebek, J. Jr Angew. Chem. Int. Edn Engl. 32, 1699–1701 (1993).
Valdés, C., Spitz, U. P., Toledo, L., Kubik, S. & Rebek, J. Jr J. Am. Chem. Soc. 117, 12733–12745 (1995).
Meissner, R. S., de Mendoza, J. & Rebek, J. Jr Science 270, 1485–1488 (1995).
Kang, J. & Rebek, J. Jr Nature 382, 239–241 (1996).
Kelly, T. R., Meghani, P. & Ekkundi, V. S. Tetrahed. Lett. 31, 3381–3384 (1990).
Meissner, R., Garcias, X., Mecozzi, S. & Rebek, J. Jr J. Am. Chem. Soc. (in the press).
Kirby, A. J. Adv. Phys. Org. Chem. 17, 183–278 (1980).
Mock, W. L., Irra, T. A., Wepsiec, J. P. & Adhya, M. J. Org. Chem. 54, 5302–5308 (1989).
McCurdy, A., Jimenez, L., Stauffer, D. A. & Dougherty, D. A. J. Am. Chem. Soc. 114, 10314–10321 (1992).
Hilvert, D., Hill, K. W. & Auditor, M. T. M. J. Am. Chem. Soc. 111, 9261–9263 (1989).
Grieco, P. A., Garner, P. & He, Z.-M. Tetrahedr. Lett. 24, 1897–1900 (1983).
Breslow, R., Maitra, U. & Rideout, D. Tetrahedr. Lett. 24, 1901–1904 (1983).
Braun, R., Schuster, F. & Sauer, J. Tetrahedr. Lett. 27, 1285–1288 (1986).
Singh, V. K., Raju, B. N. S. & Deota, P. T. Synth. Commun. 18, 567–574 (1988).
Grieco, P. A., Kaufman, M. D., Daeuble, J. F. & Saito, N. J. Am. Chem. Soc. 118, 2095–2096 (1996).
Breslow, R. & Guo, T. J. Am. Chem. Soc. 110, 5613–5617 (1988).
Walter, C. J., Anderson, H. L. & Sanders, J. K. M. J. Chem. Soc. Chem. Commun. 459–460 (1993).
Mohamadi, F. et al. J. Comput. Chem. 11, 440–467 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kang, J., Rebek, J. Acceleration of a Diels–Alder reaction by a self-assembled molecular capsule. Nature 385, 50–52 (1997). https://doi.org/10.1038/385050a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/385050a0
This article is cited by
-
Mimicry of the proton wire mechanism of enzymes inside a supramolecular capsule enables β-selective O-glycosylations
Nature Chemistry (2022)
-
Chemical reactivity under nanoconfinement
Nature Nanotechnology (2020)
-
Strategies for binding multiple guests in metal–organic cages
Nature Reviews Chemistry (2019)
-
Supramolecular synthesis of coumarin derivatives catalyzed by a coordination-assembled cage in aqueous solution
Science China Chemistry (2019)
-
Reversible chromism of spiropyran in the cavity of a flexible coordination cage
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.