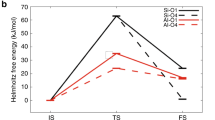
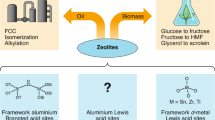
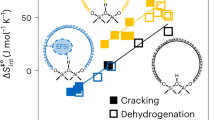
Abstract
ZEOLITES derive their catalytic activity from the strong acidity of protons attached to the negatively charged aluminosilicate frame-work, which makes the materials excellent proton donors. Unlike zeolites, the aluminophosphate molecular sieves1,2 are built from alternating AlO−4 and PO+4 tetrahedra and are thus electrically neutral. Much attention has therefore been devoted to the generation of Bronsted acidity in these materials by introducing heteroatoms, such as Si, Mg, Fe, Co or Zn, to produce negatively charged frameworks3–6. Similar arguments apply to gallophosphate molecular sieves7–10, of which cloverite9,10 is a remarkable example. This extra-large-pore material contains pore openings in the form of a four-leafed clover, defined by a ring of 20 gallium and phosphorus atoms, some of which are linked to terminal hydroxyl groups. Here we use NMR, X-ray photoelectron spectroscopy (ESCA) and infrared spectroscopy to show that the P–OH groups in cloverite are localized versions of those in solid phosphoric acid, H3PO4. Cloverite is thus a strong Bronsted acid even though no heteroatoms are present in its framework.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Wilson, S. T., Lok, B. M. & Flanigen, E. M. US Patent No. 4310440 (1982).
Wilson, S. T., Lok, B. M., Messina, C. A., Cannan, T. R. & Flanigen, E. M. J. Am. chem. Soc. 104, 1146–1147 (1982).
Lok, B. M. et al. US Patent No. 4440871 (1984).
Wilson, S. T. & Flanigen, E. M. European Patent application No. 132708 (1984).
Lok, B. M. T. et al. European Patent application No. 159624 (1985).
Lok, B. M. T., Marcus, B. K. & Flanigen, E. M. European Patent application No. 158350 (1985).
Parise, J. B. J. chem. Soc., chem. Commun. 606–607 (1985).
Yang, G., Feng, S. & Xu, R. J. chem. Soc., chem. Commun. 1254–1255 (1987).
Estermann, M., McCusker, L. B., Bärlocher, C., Merrouche, A. & Kessler, H. Nature 352, 320–323 (1991).
Merrouche, A. et al. Zeolites 12, 226–232 (1992).
Barr, T. L., Kramer, B., Shah, S. I., Ray, M. & Greene, J. E. Mater. Res. Soc. Proc. 47, 205–234 (1985).
Barr, T. L. CRC Crit. Rev. analyt. Chem. 22, 113–179, 229–325 (1991).
Barr, T. L. & Liu, Y. L. J. phys. Chem. Solids 50, 657–664 (1989).
Barr, T. L. J. Vac. Sci. and Technol. A9, 1793–1805 (1991).
Barr, T. L. & Brundle, C. R. Phys. Rev. B46, 9199–9204 (1992).
Practical Surface Analysis 2nd edn Vol. 1 (eds Briggs, D. & Seah, M. P.) 598–625 (Wiley, Chichester. 1990).
Barr, T. L. Zeolites 10, 760–765 (1990).
Wells, A. F. Structural Inorganic Chemistry 5th edn, 375 and 859 (Clarendon, Oxford, 1987).
Hair, M. L. & Hertl, W. J. phys. Chem. 74, 91–95 (1970).
Schwarzmann, E. & Sparr, H. Z. Naturf. 23B, 767–770 (1968).
Paques-Ledent, M. Th. & Tarte, P. Spectrochim. Acta 25A, 1115–1125 (1968).
Barr, T. L. & Lishka, M. A. J. Am. chem. Soc. 108, 3178–3186 (1986).
He, H., Barr, T. L. & Klinowski, J. J. phys. Chem. (submitted).
Slichter, C. P. Principles of Magnetic Resonance 3rd edn (Springer, New York, 1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barr, T., Klinowski, J., He, H. et al. Evidence for strong acidity of the molecular sieve cloverite. Nature 365, 429–431 (1993). https://doi.org/10.1038/365429a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/365429a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.