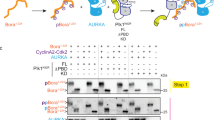
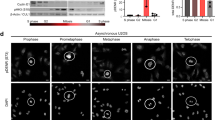
Abstract
THE G2-M phase transition in eukaryotes is regulated by the synergistic and opposing activities of a cascade of distinct protein kinases and phosphatases. This cascade converges on Cdc2, a serine/threonine protein kinase required for entry into mitosis (reviewed in ref. 1). In the fission yeast Schizosaccharomyces pombe, inactivation of the Cdc2/cyclin B complex is achieved by phosphorylation of tyrosine 15 by Weel (refs 2, 3). The action of the Weel kinase is opposed by the action of the Cdc25 phosphatase, which dephosphorylates Cdc2 on tyrosine 15, thereby activating the Cdc2/cyclin B complex4–9. Much less is known about the regulatory signals upstream of cdc25 and wee1. Genetics indicate that the mitotic inducer nim1/cdr1 acts upstream of wee1, possibly as a negative regulator of wee1 (refs 10, 11). To characterize the nim1/cdr1 protein (Nim1), we have overproduced it in both bacterial and baculoviral expression systems. We report that Nim1 possesses intrinsic serine-kinase, threonine-kinase and tyrosine-kinase activities. Co-expression of the Nim1 and Wee1 kinases in insect cells results in the phosphorylation of Weel and therefore a shift in its electrophoretic mobility on SDS–polyacrylamide gels. When Weel is phosphorylated, its ability to phosphorylate Cdc2 on tyrosine 15 is inhibited; treatment with phosphatase restores this kinase activity. Furthermore, purified bacterially produced Nim1 kinase directly phosphorylates and inactivates Weel in vitro. These results show that nim1/cdr1 functions as a positive regulator of mitosis by directly phosphorylating and inactivating the mitotic inhibitor Weel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nurse, P. Nature 344, 503–507 (1990).
Parker, L. L. et al. EMBO J. 10, 1255–1263 (1991).
Parker, L. L., Atherton-Fessler, S. & Piwnica-Worms, H. Proc. natn. Acad. Sci. U.S.A. 89, 2917–2921 (1992).
Strausfeld, U. et al. Nature 351, 242–245 (1991).
Gautier, J., Solomon, M. J., Booher, R. N., Bazan, J. F. & Kirschner, M. W. Cell 67, 197–211 (1991).
Galaktionov, K. & Beach, D. Cell 67, 1181–1194 (1991).
Kumagai, A. & Dunphy, W. G. Cell 64, 903–914 (1991).
Lee, W. S. et al. Molec Biol. Cell 3, 73–84 (1992).
Millar, J. B. A., McGowan, C. H., Lenaers, G., Jones, R. & Russell, P. EMBO J. 10, 4301–4309 (1991).
Feilotter, H., Nurse, P. & Young, P. G. Genetics 127, 309–318 (1991).
Russell, P. & Nurse, P. Cell 49, 569–576 (1987).
Piwnica-Worms, H. in Current Protocols in Molecular Biology (ed. Ausubel, F.) (Greene, New York, 1990).
Parker, L. L. & Piwnica-Worms, H. Science 257, 1955–1957 (1992).
Russell, P., Moreno, S. & Reed, S. Cell 57, 295–303 (1989)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Parker, L., Walter, S., Young, P. et al. Phosphorylation and inactivation of the mitotic inhibitor Weel by the nim1/cdr1 kinase. Nature 363, 736–738 (1993). https://doi.org/10.1038/363736a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/363736a0
This article is cited by
-
Regulation of Chk1
Cell Division (2009)
-
Polar gradients of the DYRK-family kinase Pom1 couple cell length with the cell cycle
Nature (2009)
-
A spatial gradient coordinates cell size and mitotic entry in fission yeast
Nature (2009)
-
The regulatory β-subunit of protein kinase CK2 regulates cell-cycle progression at the onset of mitosis
Oncogene (2008)
-
Yeast polo-like kinases: functionally conserved multitask mitotic regulators
Oncogene (2005)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.