Abstract
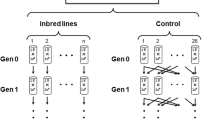
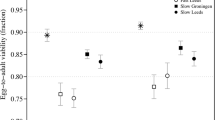
THE mutation rate per genome for local affecting fitness is crucial in theories of the evolution of sex and recombination1,2 and of outbreeding mechanisms3. Mutational variation in fitness may also be important in the evolution of mate choice in animals2,4,5. No information is available on the rate at which spontaneous mutations with small effects on fitness arise, although viability (probability of survival to adulthood) has been studied in Drosophila melanogaster6–9. These experiments involved the accumulation of spontaneous mutations in the virtual absence of natural selection, in a set of independently maintained lines with a common origin. The rates of decline in mean and increase in variance among lines permit estimation of limits to the mean number of new mutations arising per generation (U) and the average homozygous effect of a new mutation of minor effect (s)7,9,10. For the second chromosome of D. melanogaster, the value of U is at least 0.17 (ref. 7), and (1 – h)s is less than 0.02, where hsis the average decline in fitness of heterozygotes. As the second chromosome is about 40% of the genome, these data indicate a mutation rate per haploid genome of at least 0.42 for viability. Here we present similar data on the effects of homozygous spontaneous mutations on a measure of fitness in D. melanogaster.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Crow, J. F. in Mathematical Topics in Population Genetics (ed. Kojima, K.-I.) 128–177 (Springer, Berlin, 1970).
Kondrashov, A. S. Nature 336, 435–440 (1988).
Charlesworth, D. & Charlesworth, B. A. Rev. Ecol. Syst. 18, 237–268 (1987).
Charlesworth, B. in Sexual Selection: Testing the Alternatives (eds Bradbury, J. W. & Andersson, M. B.) 21–40 (Wiley, Chichester, 1987).
Kirkpatrick, M. & Ryan, M. J. Nature 350, 33–38 (1991).
Mukai, T. Genetics 50, 1–19 (1964).
Mukai, T., Chigusa, S. I., Mettler, L. E. & Crow, J. F. Genetics 72, 335–355 (1972).
Ohnishi, O. Genetics 87, 529–545 (1977).
Crow, J. F. & Simmons, M. J. in The Genetics and Biology of Drosophila, Vol. 3C (eds Ashburner, M., Carson, H. L. & Thomson, J. N.) 1–35 (Academic, London, 1983).
Bateman, A. J. Int. J. Radiat. Biol. 2, 170–180 (1959).
Sved, J. A. Genet. Res. Camb. 18, 97–105 (1971).
Ashburner, M. Drosophila. A Laboratory Handbook (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 1989).
Sokal, R. R. & Rohlf, F. J. Biometry (Freeman, San Francisco, 1981).
Simmons, M. J., Preston, C. R. & Engels, W. R. Genetics 94, 467–475 (1980).
Mukai, T. & Yamazaki, T. Genetics 69, 385–398 (1971).
Yoshimaru, H. & Mukai, T. Jap. J. Genet. 60, 307–334 (1985).
Mukai, T. Genetics 61, 749–761 (1969).
Charlesworth, B., Charlesworth, D. & Morgan, M. T. Nature 347, 380–382 (1990).
Charlesworth, B. & Charlesworth, D. Heredity 54, 71–84 (1985).
Lindsley, D. L. & Grell, E. H. Genetic Variations of Drosophila melanogaster (Carnegie Institute, Washington, 1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Houle, D., Hoffmaster, D., Assimacopoulos, S. et al. The genomic mutation rate for fitness in Drosophila. Nature 359, 58–60 (1992). https://doi.org/10.1038/359058a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/359058a0
This article is cited by
-
Genome-wide autozygosity is associated with lower general cognitive ability
Molecular Psychiatry (2016)
-
The genetics of inbreeding depression
Nature Reviews Genetics (2009)
-
Analysis and implications of mutational variation
Genetica (2009)
-
The population genetics of ecological specialization in evolving Escherichia coli populations
Nature (2000)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.