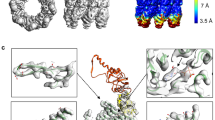
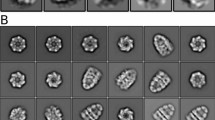
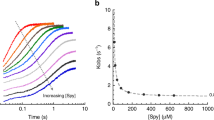
Abstract
Folding of two monomeric enzymes mediated by groE has been reconstituted in vitro. The groEL protein stabilizes the polypeptides in a conformation resembling the 'molten globule' state. Mg–ATP and groES then promote the acquisition of ordered tertiary structure at the surface of groEL. Folding requires the hydrolysis of about 100 ATP molecules per protein monomer. This active process of surface-mediated chain folding might represent a general mechanism for the formation of protein structure in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fischer, G. & Schmid, F. X. Biochemistry 29, 2206–2212 (1990).
Freedman, R. B. Cell 57, 1069–1072 (1989).
Ellis, R. J. & van der Vies, S. M. A. Rev. Biochem. 60, 327–347 (1991).
Ellis, R. J. Nature 328, 378–379 (1987).
Rothman, J. E. Cell 59, 591–601 (1989).
Pelham, H. R. B. Nature 332, 776–777 (1988).
Hartl, F.-U. Semin. Immun. (in the press).
Hemmingsen, S. M. et al. Nature 333, 330–334 (1988).
Georgopoulos, C., Hendrix, R., Casjens, S. R. & Kaiser, A. D. J. molec. Biol. 76, 45–60 (1973).
Sternberg, N. J. molec. Biol. 76, 25–44 (1973).
Hendrix, R. W. J. molec. Biol. 129, 375–392 (1979).
Bochkareva, E. S., Lissin, N. M. & Girshowich, A. S. Nature 336, 254–257 (1988).
Goloubinoff, P., Christeller, J. T., Gatenby, A. A. & Lorimer, G. H. Nature 342, 884–889 (1989).
Van Dyk, T. K., Gatenby, A. A. & La Rossa, R. A. Nature 342, 451–453.
Lecker, S. et al. EMB0 J. 8, 2703–2709 (1989).
Laminet, A. A., Ziegelhoffer, T., Georgopoulos, C. & Plückthun, A. EMB0 J. 9, 2315–2319 (1990).
Buchner, J. et al. Biochemistry 30, 1586–1591 (1991).
Barraclough, R. & Ellis, R. J. Biochim. biophys. Acts 608, 19–31 (1980).
Musgrove, J. E., Johnson, R. A. & Ellis, R. J. Eur. J. Biochem. 163, 529–534 (1987).
Gatenby, A. A. & Ellis, R. J. A. Rev. Cell Biol. 6, 125–149 (1990).
McMullin, T. W. & Hallberg, R. L. Molec. cell. Biol. 8, 371–380 (1988).
Reading, D. S., Hallberg, R. L. & Myers, A. M. Nature 337, 655–659 (1989).
Cheng, M. Y. et al. Nature 337, 620–625 (1989).
Ostermann, J., Horwich, A. L., Neupert, W. & Hartl, F.-U. Nature 341, 125–130 (1989).
Cheng, M. Y., Hartl, F.-U. & Horwich, A. L. Nature 348, 455–458 (1990).
Chandrasekhar, G. N., Tilly, K., Woofford, C., Hendrix, R. & Georgopoulos, C. J. biol. Chem. 261, 12414–12419 (1986).
Lubben, T. H., Gatenby, A. A., Donaldson, G. K., Lorimer, G. H. & Viitanen, P. V. Proc. natn. Acad. Sci. U.S.A. 87, 7683–7687 (1990).
Viitanen, P. V. et al. Biochemistry 29, 5665–5670 (1990).
Ptitsyn, O. B., Pain, R. H., Semisotnov, G. V., Zerovnik, E. & Razgulyaev, O. I. FEBS Lett. 262, 20–24 (1990).
Ewbank, J. J. & Creighton, T. E. Nature 350, 518–520 (1991).
Kuwajima, K. Proteins 6, 87–103 (1989).
Creighton, T. E. Biochem. J. 270, 1–16 (1990).
Kumar, A. A., Blankenship, D. T., Kaufman, B. T. & Freisheim, J. H. Biochemistry 19, 667–678 (1980).
Matthews, D. A. et al. J. biol. Chem. 260, 381–391 (1985).
Touchette, N. A., Perry, K. M. & Matthews, C. R. Biochemistry 25, 5445–5452 (1986).
Waxman, L. & Goldberg, A. L. Science 232, 500–503 (1986).
Ostoa-Saloma, P., Ramirez, J. & Perez-Montfort, R. Biochim. biophys. Acta 1041, 140–152 (1990).
Jaenicke, R. Prog. Biophys. Molec. Biol. 49, 117–237 (1987).
Ploegman, J. H. et al. Nature 273, 124–129 (1978).
Tandon, S. & Horowitz, P. M. J. biol. Chem. 261, 15615–15681 (1986).
Hohn, T., Hohn, B., Engel, A., Wortz, M. & Smith, P. R. J. molec. Biol. 129, 359–373 (1979).
Huang, S. et al. Biochemistry 28, 471–478 (1989).
Semisotnov, G. V. et al. FEBS Lett. 224, 9–13 (1987).
Tandon, S. & Horowitz, P. M. J. biol. Chem. 264, 9859–9866 (1989).
Tandon, S. & Horowitz, P. M. J. biol. Chem. 265, 5967–5970 (1990).
Garvey, E. P., Swank, J. & Matthews, C. R. Proteins struct. fund. Genet. 6, 259–266 (1989).
Flynn, G. C., Chapell, T. G. & Rothman, J. E. Science 245, 385–390 (1989).
Kang, P.-J. et al. Nature 348, 137–143 (1990).
Scherer, P. E., Krieg, U. C., Hwang, S. T., Vestweber, D. & Schatz, G. EMB0 J. 9, 4315–4322 (1990).
Fayet, O., Louran, J. M. & Georgopoulos, C. Molec. gen. Genet. 202, 35–445 (1986).
Vachereau, A. Analyt. Biochem. 179, 206–208 (1989).
Jaenicke, R. & Rudolph, R. in Protein Structure, a Practical Approach (ed. Creighton, T. E.) 191–222 (IRL, Oxford, (1989).
Lill, R., Dowhan, W. & Wickner, W. Cell 60, 271–280 (1990).
Lehrer, S. S. Biochemistry 10, 3254–3263 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martin, J., Langer, T., Boteva, R. et al. Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature 352, 36–42 (1991). https://doi.org/10.1038/352036a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/352036a0
This article is cited by
-
How soluble misfolded proteins bypass chaperones at the molecular level
Nature Communications (2023)
-
The effects of cold stress on Mytilus species in the natural environment
Cell Stress and Chaperones (2020)
-
A look back at the molten globule state of proteins: thermodynamic aspects
Biophysical Reviews (2019)
-
The TRiC chaperonin controls reovirus replication through outer-capsid folding
Nature Microbiology (2018)
-
Evolutionarily Related Dihydrofolate Reductases Perform Coequal Functions Yet Show Divergence in Their Trajectories
The Protein Journal (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.