Key Points
-
Different chronic pain states generate different neurochemical changes in sensory fibres and the spinal cord. Understanding these distinct molecular signatures might be the key to effective pain control.
-
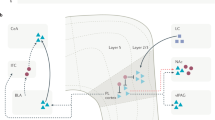
Sensory fibres carry information from the skin and most internal tissues to the spinal cord. Anatomically, there are two broad groups of sensory fibre: myelinated A fibres and smaller diameter, unmyelinated C fibres. Most C fibres are polymodal nociceptors that respond to all forms of noxious stimulation.
-
C fibres can be divided into two groups. One group expresses receptors for GDNF, and terminates almost exclusively within the deeper parts of the substantia gelatinosa of the spinal cord. The other group synthesizes peptides such as substance P, expresses the NGF receptor TrkA and terminates more superficially within the dorsal horn.
-
Noxious stimulation changes the phenotype of sensory neurons. In part, these changes are caused by a change in the levels of growth factors released from the injury area. NGF has been shown to regulate the behavioural sensitivity to pain. GDNF-sensitive sensory fibres might be involved in establishing chronic pain states.
-
Pain has sensory and affective qualities. The spinothalamic pathway, which originates primarily from neurons in the neck of the dorsal horn and terminates within the thalamus, is thought to convey the sensory qualities of the stimulus.
-
The spinoparabrachial pathway, which derives largely from lamina I neurons of the dorsal horn that express the substance P/neurokinin-1 (NK1) receptor, terminates within the parabrachial nuclei and periaqueductal grey. In turn, these areas project on areas such as the hypothalamus and amygdala that modulate the affective dimensions of pain and control autonomic activity.
-
Selective ablation of lamina I neurons that express the NK1 receptor revealed that this population is pivotal in the signalling of pain and the central maintenance of hyperalgesia.
-
Selective knockout of the NK1 receptor blunts the rewarding effect of morphine while leaving its analgesic effect largely intact. So NK1-receptor antagonists could counteract problems of morphine dependency in the clinic.
Abstract
Pain is necessary for survival, but persistent pain can result in anxiety, depression and a reduction in the quality of life. The discriminative and affective qualities of pain are both thought to be regulated in an activity-dependent fashion. Recent studies have identified cells and molecules that regulate pain sensitivity and the parallel pathways that distribute nociceptive information to limbic or sensory areas of the forebrain. Here, we emphasize the cellular and neurobiological consequences of pain, especially those that are involved in the generation and maintenance of chronic pain. These new insights into pain processing will significantly alter our approach to pain control and the development of new analgesics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hunt, S. P. & Rossi, J. Peptide- and non-peptide-containing unmyelinated primary afferents: the parallel processing of nociceptive information . Phil. Trans. R. Soc. Lond. B 308, 283– 289 (1985). PubMed
Willis, W. D. & Westlund, K. N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 14, 2–31 (1997 ).
Bernard, J. F. & Bandler, R. Parallel circuits for emotional coping behaviour: new pieces in the puzzle. J. Comp. Neurol. 401, 429–436 (1998).
Craig, A. D. in The Emotional Motor System. Progress in Brain Research Vol. 107 (eds Holstege, G., Saper, C. & Bandler, R.) 225– 242 (Elsevier, New York, 1996).
Villanueva, L., Bouhassira, D. & Le Bars, D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain 67, 231–240 ( 1996).
Lima, D., Mendes-Ribeiro, J. A. & Coimbra, A. The spino-latero-reticular system of the rat: projections from the superficial dorsal horn and structural characterization of marginal neurons involved. Neuroscience 45, 137– 152 (1991).
Urban, M. O. & Gebhart, G. F. Supraspinal contributions to hyperalgesia. Proc. Natl Acad. Sci. USA 96, 7687–7692 (1999).
Dubner, R. & Ren, K. Endogenous mechanisms of sensory modulation . Pain 6, S45–S53 (1999 ). PubMed
Basbaum, A. I. & Fields, H. L. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 7, 309–338 ( 1984).
Honore, P. et al. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience 98, 585– 598 (2000).
Hokfelt, T., Zhang, X. & Wiesenfeld-Hallin, Z. Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 17, 22–30 (1994).
Chizh, B. A., Dickenson, A. H. & Wnendt, S. The race to control pain: more participants, more targets . Trends Pharmacol. Sci. 20, 354– 357 (1999).
Hunt, S. P., Pini, A. & Evan, G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation . Nature 328, 632–634 (1987).
Williams, S., Evan, G. I. & Hunt, S. P. Changing patterns of c-fos induction in spinal neurons following thermal cutaneous stimulation in the rat. Neuroscience 36, 73–81 ( 1990).
Fields, H. in Molecular Neurobiology of Pain (ed. Borsook, D.) 307– 317 (IASP, Seattle, 1997).
Yaksh, T. L. Spinal systems and pain processing: development of novel analgesic drugs with mechanistically defined models. Trends Pharmacol. Sci. 20, 329–337 (1999).
Snider, W. D. & McMahon, S. B. Tackling pain at the source: new ideas about nociceptors. Neuron 20, 629–632 (1998).
Michaelis, M., Habler, H. J. & Jaenig, W. Silent afferents: a separate class of primary afferents? Clin. Exp. Pharmacol. Physiol. 23, 99– 105 (1996).
Xu, G. Y., Huang, L. Y. & Zhao, Z. Q. Activation of silent mechanoreceptive cat C and A sensory neurons and their substance P expression following peripheral inflammation . J. Physiol. 528, 339– 348 (2000).
Nagy, J. I. & Hunt, S. P. Fluoride-resistant acid phosphatase-containing neurons in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience 7, 89– 97 (1982).
Ribeiro-Da-Silva, A., Castro-Lopes, J. M. & Coimbra, A. Distribution of glomeruli with fluoride-resistant acid phosphatase (FRAP)-containing terminals in the substantia gelatinosa of the rat. Brain Res. 377, 323– 329 (1986).
Guo, A., Vulchanova, L., Wang, J., Li, X. & Elde, R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur. J. Neurosci. 11, 946–958 (1999).
Silverman, J. D. & Kruger, L. Lectin and neuropeptide labeling of separate populations of dorsal root ganglion neurons and associated 'nociceptor' thin axons in rat testis and cornea whole-mount preparations . Somatosens. Res. 5, 259– 267 (1988).
Bennett, D. L. et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 18, 3059–3072 (1998).
Ribeiro-da-Silva, A. & Coimbra, A. Capsaicin causes selective damage to type I synaptic glomeruli in rat substantia gelatinosa . Brain Res. 290, 380–383 (1984).
Averill, S., McMahon, S. B., Clary, D. O., Reichardt, L. F. & Priestley, J. V. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur. J. Neurosci. 7, 1484– 1494 (1995).
Cuello, A. C., Ribeiro-da-Silva, A., Ma, W., De Koninck, Y. & Henry, J. L. Organization of substance P primary sensory neurons: ultrastructural and physiological correlates. Regul. Pept. 46, 155–164 (1993).
Michael, G. J. & Priestley, J. V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy . J. Neurosci. 19, 1844– 1854 (1999).
Caterina, M. J. et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816– 824 (1997).References 29 and 51 imply that there might be pharmacological targets for treating pain that are preferentially expressed by peripheral sensory neurons.
Bennett, D. L., Koltzenburg, M., Priestley, J. V., Shelton, D. L. & McMahon, S. B. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur. J. Neurosci. 10, 1282–1291 (1998).
Oddiah, D., Anand, P., McMahon, S. B. & Rattray, M. Rapid increase of NGF, BDNF and NT-3 mRNAs in inflamed bladder. Neuroreport 9, 1455–1458 (1998).
Koltzenburg, M., Bennett, D. L., Shelton, D. L. & McMahon, S. B. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur. J. Neurosci. 11, 1698–1704 (1999).
Csillik, B. Nerve growth factor regulates central terminals of primary sensory neurons . Z. Mikrosk Anat. Forsch. 98, 11– 16 (1984).
Fitzgerald, M., Wall, P. D., Goedert, M. & Emson, P. C. Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section. Brain Res. 332, 131 –141 (1985).
Woolf, C. J., Shortland, P. & Coggeshall, R. E. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 355, 75– 78 (1992).
Bennett, D. L., French, J., Priestley, J. V. & McMahon, S. B. NGF but not NT-3 or BDNF prevents the A fiber sprouting into lamina II of the spinal cord that occurs following axotomy. Mol. Cell. Neurosci. 8, 211–220 ( 1996).
Boucher, T. J. et al. Potent analgesic effects of GDNF in neuropathic pain states . Science 290, 124–127 (2000). PubMed
Malmberg, A. B., Chen, C., Tonegawa, S. & Basbaum, A. I. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science 278, 279–283 ( 1997).References 37 and 38 identify novel targets for treating and understanding the mechanisms that give rise to neuropathic pain.
Bozic, C. R., Lu, B., Hopken, U. E., Gerard, C. & Gerard, N. P. Neurogenic amplification of immune complex inflammation . Science 273, 1722–1725 (1996).
De Felipe, C. et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392, 394–397 (1998).The first demonstration of the wide-ranging roles of substance P in behaviour using gene knockout.
McMahon, S. B., Bennett, D. L., Priestley, J. V. & Shelton, D. L. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA–IgG fusion molecule. Nature Med. 1, 774–780 ( 1995).
Neumann, S., Doubell, T. P., Leslie, T. & Woolf, C. J. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature 384, 360 –364 (1996).
Swett, J. E. & Woolf, C. J. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J. Comp. Neurol. 231, 66–77 (1985).
Janig, W. & Koltzenburg, M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J. Auton. Nerv. Syst. 30, S89–S96 (1990).
Cervero, F. & Tattersall, J. E. Somatic and visceral inputs to the thoracic spinal cord of the cat: marginal zone (lamina I) of the dorsal horn. J. Physiol. (Lond.) 388, 383– 395 (1987).
Morgan, C., Nadelhaft, I. & deGroat, W. C. The distribution within the spinal cord of visceral primary afferent axons carried by the lumbar colonic nerve of the cat. Brain Res. 398, 11–17 ( 1986).
Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531– 543 (1998).
Davis, J. B. et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183– 187 (2000).
Caterina, M. J. et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306– 313 (2000).
Caterina, M. J., Rosen, T. A., Tominaga, M., Brake, A. J. & Julius, D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398, 436–441 (1999).
Akopian, A. N., Sivilotti, L. & Wood, J. N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature 379, 257–262 (1996).
Sangameswaran, L. et al. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. J. Biol. Chem. 272, 14805–14809 ( 1997).
Novakovic, S. D. et al. Distribution of the tetrodotoxin-resistant sodium channel PN3 in rat sensory neurons in normal and neuropathic conditions. J. Neurosci. 18, 2174–2187 (1998).
Cummins, T. R. et al. A novel persistent tetrodotoxin-resistant sodium current In SNS-null and wild-type small primary sensory neurons. J. Neurosci. 19, RC43 (1999).
Waxman, S. G., Dib-Hajj, S., Cummins, T. R. & Black, J. A. Sodium channels and pain. Proc. Natl Acad. Sci. USA 96, 7635–7639 (1999).
Tate, S. et al. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nature Neurosci. 1, 653–655 (1998).
Coward, K. et al. Immunolocalization of SNS/PN3 and NaN/SNS2 sodium channels in human pain states. Pain 85, 41– 50 (2000).
Okuse, K. et al. Regulation of expression of the sensory neuron-specific sodium channel SNS in inflammatory and neuropathic pain. Mol. Cell. Neurosci. 10, 196–207 ( 1997).
Akopian, A. N. et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nature Neurosci. 2, 541–548 (1999).
Calza, L. et al. Peptide plasticity in primary sensory neurons and spinal cord during adjuvant-induced arthritis in the rat: an immunocytochemical and in situ hybridization study. Neuroscience 82, 575–589 (1998).
Zhang, X., Bean, A. J., Wiesenfeld-Hallin, Z., Xu, X. J. & Hokfelt, T. Ultrastructural studies on peptides in the dorsal horn of the rat spinal cord. III. Effects of peripheral axotomy with special reference to galanin. Neuroscience 64, 893–915 (1995).
Schwei, M. J. et al. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J. Neurosci. 19, 10886–10897 (1999). This paper describes a mouse model of bone cancer pain and suggests a mechanism by which bone cancer pain is generated.
Honore, P. et al. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nature Med. 6, 521– 528 (2000).
Meller, S. T., Dykstra, C., Grzybycki, D., Murphy, S. & Gebhart, G. F. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology 33, 1471–1478 (1994).
Peschanski, M., Mantyh, P. W. & Besson, J. M. Spinal afferents to the ventrobasal thalamic complex in the rat: an anatomical study using wheat-germ agglutinin conjugated to horseradish peroxidase. Brain Res. 278, 240–244 (1983).
Mantyh, P. W. The spinothalamic tract in the primate: a re-examination using wheatgerm agglutinin conjugated to horseradish peroxidase. Neuroscience 9, 847–862 (1983).
Dado, R. J., Katter, J. T. & Giesler, G. J. J. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. Responses to innocuous and noxious mechanical and thermal stimuli. J. Neurophysiol. 71 , 981–1002 (1994).
Katter, J. T., Dado, R. J., Kostarczyk, E. & Giesler, G. J. J. Spinothalamic and spinohypothalamic tract neurons in the sacral spinal cord of rats. I. Locations of antidromically identified axons in the cervical cord and diencephalon. J. Neurophysiol. 75, 2581 –2605 (1996).
Marshall, G. E., Shehab, S. A., Spike, R. C. & Todd, A. J. Neurokinin-1 receptors on lumbar spinothalamic neurons in the rat. Neuroscience 72, 255–263 (1996).
Todd, A. J., McGill, M. M. & Shehab, S. A. Neurokinin 1 receptor expression by neurons in laminae I, III and IV of the rat spinal dorsal horn that project to the brainstem . Eur. J. Neurosci. 12, 689– 700 (2000).
Ding, Y. Q., Takada, M., Shigemoto, R. & Mizumo, N. Spinoparabrachial tract neurons showing substance P receptor-like immunoreactivity in the lumbar spinal cord of the rat. Brain Res. 674 , 336–340 (1995). References 69 – 71 qualitatively identify the spinoparabrachial pathway as the main ascending pain pathway in rodents. It originates largely from lamina I/NK1-positive neurons.
Bernard, J. F., Bester, H. & Besson, J. M. Involvement of the spino-parabrachio-amygdaloid and hypothalamic pathways in the autonomic and affective emotional aspects of pain. Prog. Brain Res. 107, 243– 255 (1996).This paper demonstrates that parabrachial neurons are noci-specific and concerned predominantly with the intensity of pain rather than its location or nature.
Bester, H., Chapman, V., Besson, J. M. & Bernard, J. F. Physiological properties of the lamina I spinoparabrachial neurons in the rat. J. Neurophysiol. 83, 2239– 2259 (2000).
Doyle, C. A. & Hunt, S. P. Substance P receptor (neurokinin-1)-expressing neurons in lamina I of the spinal cord encode for the intensity of noxious stimulation: a c-Fos study in rat. Neuroscience 89, 17–28 (1999).
Mantyh, P. W. et al. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science 278, 275–279 (1997).
Nichols, M. L. et al. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science 286, 1558 –1561 (1999).References 75 and 76 describe the use of a suicide-ligand approach to selectively target and destroy cells involved in the ascending conduction of pain.
Bester, H., De Felipe C. D. & Hunt, S. P. The substance P receptor is essential for the full expression of noxious inhibitory controls in the mouse. J. Neurosci. (in the press).
Hirakawa, N., Tershner S. A., Fields, H. L. & Manning, B. H. Bi-directional changes in affective state elicited by manipulation of medullary pain-modulatory circuitry. Neuroscience 100, 861–871 (2000).
Culman, J. & Unger, T. Central tachykinins: mediators of defence reaction and stress reactions. Can. J. Physiol. Pharmacol. 73, 885–891 ( 1995).
Rupniak, N. M. et al. Pharmacological blockade or genetic deletion of substance P (NK(1)) receptors attenuates neonatal vocalisation in guinea-pigs and mice . Neuropharmacology 39, 1413– 1421 (2000).
Murtra, P., Sheasby, A. M., Hunt, S. P. & De Felipe, C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature 405, 180–183 (2000).The first demonstration that the rewarding and analgesic properties of opiates are separable following knockout of the NK1-receptor gene.
Laird, J. M. et al. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience 98, 345–352 (2000).
Kramer, M. S. et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281, 1640–1645 (1998). This paper presents both preclinical and clinical data indicating that NK1-receptor antagonists might be useful in the treatment of anxiety and depression.
Treede, R. D., Kenshalo, D. R., Gracely, R. H. & Jones, A. K. The cortical representation of pain. Pain 79, 105–111 (1999).
Price, D. D. Psychological and neural mechanisms of the affective dimension of pain. Science 288, 1769–1772 ( 2000).
Rainville, P., Carrier, B., Hofbauer, R. K., Bushnell, M. C. & Duncan, G. H. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82, 159–171 (1999).
Rainville, P., Duncan, G. H., Price, D. D., Carrier, B. & Bushnell, M. C. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277, 968–971 (1997). References 86 and 87 identify the sensory and affective dimensions of pain as being separable and dependent, in part, on different brain circuits.
Flor, H. et al. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375, 482 –484 (1995).
Larbig, W. et al. Evidence for a change in neural processing in phantom limb pain patients. Pain 67, 275– 283 (1996).
Knecht, S. et al. Reorganizational and perceptional changes after amputation . Brain 119, 1213–1219 (1996).
Lenz, F. A. et al. Stimulation in the human somatosensory thalamus can reproduce both the affective and sensory dimensions of previously experienced pain. Nature Med. 1, 910–913 ( 1995).
Matthes, H. W. et al. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature 383, 819–823 (1996).
Mitchell, J. M., Basbaum, A. I. & Fields, H. L. A locus and mechanism of action for associative morphine tolerance. Nature Neurosci. 3, 47– 53 (2000).This paper shows that the amygdala-dependent mechanism of contextual sensitization to opiates is reversible by local injection of CCK-B-receptor antagonists.
Robbins, T. W. & Everitt, B. J. Drug addiction: bad habits add up. Nature 398, 567– 570 (1999).
Franklin, K. B. Analgesia and abuse potential: an accidental association or a common substrate? Pharmacol. Biochem. Behav. 59, 993– 1002 (1998).
Lumb, B. M. & Lovick, T. A. The rostral hypothalamus: an area for the integration of autonomic and sensory responsiveness. J. Neurophysiol. 70, 1570–1577 (1993).
Lovick, T. A. Midbrain and medullary regulation of defensive cardiovascular functions. Prog. Brain Res. 107, 301–313 (1996).
Koob, G. F. & Le Moal, M. Drug abuse: hedonic homeostatic dysregulation. Science 278, 52– 58 (1997).
Acknowledgements
We would like to thank Dr Herve Bester for Figures 1 and 2 and for his comments on the manuscript. S.P.H would like to thank the Wellcome Trust and the BBSRC for support during the writing of this review. P.W.M would like to thank NINDS, NIDA and VA Merit Award for support for many of the studies referred to in the text.
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
Encyclopedia of Life Sciences links
Glossary
- BRAINSTEM
-
The brainstem extends from the spinal cord and rostrally includes the midbrain and thalamus. Surrounding the narrow ventricle of the midbrain is the periaqueductal grey, an area that is crucial for fight/flight behaviour and for the associated autonomic events.
- SUBSTANTIA GELATINOSA
-
Lamina II of the spinal cord is also referred to (sometimes together with lamina I) as the substantia gelatinosa. This is the main site of termination of C-type sensory fibres, which are unmyelinated and so confer a gelatinous appearance on this region of the dorsal horn of the spinal cord when examined in unfixed preparations.
- GLOMERULUS
-
Axon terminals end in a variety of configurations within the neuropil. The most common is en passant or de passage in which axons make simple synapses as they pass dendrites or cell bodies. By contrast, some axons end in — or produce strings of — enlargements that are often packed with synaptic vesicles. These glomerular-type endings synapse with large numbers of dendrites and other axons clustered around the glomerular ending.
- SUBSTANCE P
-
Substance P is an 11-amino-acid tachykinin peptide neurotransmitter that binds preferentially to the NK1 receptor. A second tachykinin, NKA, also has high affinity for this receptor, suggesting that to refer to the NK1 receptor as 'the substance P receptor' could be misleading.
- PERIPHERAL NERVE AXOTOMY
-
Surgical cutting or crushing of the peripheral nerve. The peripheral nerve can contain a mixture of sensory, autonomic or motor fibres depending on the particular nerve.
- EXTRAVASATION
-
Refers to the leakage of plasma and plasma proteins from post-capillary venules — recognized as oedema.
- HYPERALGESIA
-
Hyperalgesia refers to the perception of a stimulus as more painful than when previously experienced.
- LAMINA I
-
The grey matter of the spinal cord has been divided up into ten laminae on the basis of the distribution of neuronal cell bodies and myelinated fibres. The most superficial is lamina I, which contains a mixture of neurons, ∼10% of which express the tachykinin NK1 receptor and project to higher centres of the brain.
- CARRAGEENAN
-
Carrageenan is an inflammatory agent extracted from seaweed that induces localized swelling and pain that peaks three hours after injection. It is used to model inflammatory pain states observed in the clinic.
- VON FREY THRESHOLDS
-
This is the point at which the stimulus — the Von Frey monofilament — produces a withdrawal response. The threshold is established by using filaments that exert a stimulus intensity that depends on their diameter.
- DORSAL ROOT GANGLION
-
The cell bodies of sensory neurons are collected together in paired ganglia that lie alongside the spinal cord. These cell bodies are surrounded by satellite glial cells, which have much in common with the Schwann cells that ensheath peripheral axons. Very few synapses have been observed in these ganglia.
- ALLODYNIA
-
Allodynia refers to the perception of a stimulus as painful when previously the stimulus was reported to be non-painful. As with hyperalgesia, this category was derived from observations on humans in which verbal reporting was used to assess pain sensitivity, and so it is very difficult to designate a change in pain sensitivity in animals as allodynic or hyperalgesic.
- COMPLETE FREUNDS ADJUVANT
-
Complete Freunds Adjuvant is an inflammatory agent that induces localized swelling and pain that peaks three days after injection. It is used to model persistent pain states observed in the clinic.
- CHUNG MODEL
-
There are several widely used neuropathic pain models. The Chung model is produced by a tight ligation of lumber dorsal roots L5 and L6 close to their exit from the dorsal root ganglion, leaving L4 intact. Stimuli applied within the peripheral cutaneous receptive field of L5 axons produce a heightened behavioural withdrawal response of the limb, suggesting increased sensitivity to previously non-noxious and noxious stimuli.
- STRESS-INDUCED ANALGESIA
-
Stress has been shown to reduce sensitivity to noxious stimulation. In mice, moderate stress produces an analgesia that is mediated by endogenous opiates, whereas more severe stress results in a non-opioid-mediated, NK1-mediated and NMDA-receptor-mediated analgesia.
Rights and permissions
About this article
Cite this article
Hunt, S., Mantyh, P. The molecular dynamics of pain control. Nat Rev Neurosci 2, 83–91 (2001). https://doi.org/10.1038/35053509
Published:
Issue Date:
DOI: https://doi.org/10.1038/35053509
This article is cited by
-
Alterations of the gut microbiota in patients with postherpetic neuralgia
AMB Express (2023)
-
Evaluation of Substance P concentrations in the blood plasma of jugular and tail vein of healthy German Simmental cows
BMC Veterinary Research (2023)
-
Hallmarks of peripheral nerve function in bone regeneration
Bone Research (2023)
-
Substance P concentrations in the blood plasma and serum of adult cattle and calves during different painful procedures and conditions – a systematic review
BMC Veterinary Research (2022)
-
Coexistence of chronic hyperalgesia and multilevel neuroinflammatory responses after experimental SCI: a systematic approach to profiling neuropathic pain
Journal of Neuroinflammation (2022)