Abstract
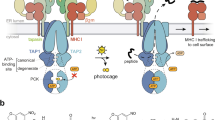
The transporter associated with antigen processing (TAP) is a member of the family of ABC transporters that translocate a large variety of substrates across membranes1. TAP transports peptides from the cytosol into the endoplasmic reticulum for binding to MHC class I molecules and for subsequent presentation to the immune system2. Here we follow the lateral mobility of TAP in living cells. TAP's mobility increases when it is inactive and decreases when it translocates peptides. Because TAP activity is dependent on substrate, the mobility of TAP is used to monitor the intracellular peptide content in vivo. Comparison of the diffusion rates in peptide-free and peptide-saturated cells indicates that normally about one-third of all TAP molecules actively translocate peptides. However, during an acute influenza infection TAP becomes fully employed owing to the production and degradation of viral proteins. Furthermore, TAP activity depends on continuing protein translation. This implies that MHC class I molecules mainly sample peptides that originate from newly synthesized proteins, to ensure rapid presentation to the immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Higgins,C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 ( 1992).
Pamer,E. & Cresswell,P. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 16, 323–358 (1998).
Vos,J. C., Spee,P., Momburg,F. & Neefjes,J. Membrane topology and dimerization of the two subunits of the transporter associated with antigen processing reveal a three-domain structure. J. Immunol. 163, 6679–6685 (1999).
Vos,J. C., Reits,E. A. J., Wojcik-Jacobs, E. & Neefjes,J. Head–head/tail–tail relative orientation of the pore-forming domains of the heterodimeric ABC transporter TAP. Curr. Biol. 10, 1–7 (2000).
Wubbolts,R. et al. Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J. Cell Biol. 135, 611–622 (1996).
Kwon,G., Axelrod,D. & Neubig,R. R. Lateral mobility of tetramethylrhodamine (TMR) labelled G protein alpha and beta gamma subunits in NG 108-15 cells. Cell Signal. 6, 663–679 ( 1994).
Schlessinger,J. et al. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc. Natl Acad. Sci. USA 73, 2409–2413 (1976).
Marguet,D. et al. Lateral diffusion of GFP-tagged H2Ld molecules and of GFP-TAP1 reports on the assembly and retention of these molecules in the endoplasmic reticulum. Immunity 11, 231– 240 (1999).
Neefjes,J. J., Momburg,F. & Hammerling, G. J. Selective and ATP-dependent translocation of peptides by the MHC-encoded transporters. Science 261, 769–771 (1993).
Fenteany,G. et al. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268, 726–731 (1995).
van Endert,P. M. et al. A sequential model for peptide binding and transport by the transporters associated with antigen processing. Immunity 1, 491–500 (1994).
Grommé,M. et al. The rational design of TAP inhibitors using peptide substrate modifications and peptidomimetics. Eur. J. Immunol. 27, 898–904 (1997).
Grommé,M. et al. Recycling MHC class I molecules and endosomal peptide loading. Proc. Natl Acad. Sci. USA 96, 10326– 10331 (1999).
Neisig,A., Wubbolts,R., Zang,X., Melief,C. & Neefjes,J. Allele-specific differences in the interaction of MHC class I molecules with transporters associated with antigen processing. J. Immunol. 156, 3196–3206 (1996).
Lehner,P. J. & Trowsdale,J. Antigen presentation: coming out gracefully. Curr. Biol. 8, R605– R608 (1998).
Ahn,K. et al. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6, 613– 621 (1997).
Hengel,H. et al. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6, 623– 632 (1997).
Livneh,E. et al. Large deletions in the cytoplasmic kinase domain of the epidermal growth factor receptor do not affect its lateral mobility. J. Cell Biol. 103, 327–331 ( 1986).
de Leij,L. et al. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 45, 6024–6033 (1985).
Saffman,P. G. & Delbruck,M. Brownian motion in biological membranes. Proc. Natl Acad. Sci. USA 72, 3111– 3113 (1975).
Cha,A., Snyder,G. E., Selvin,P. R. & Bezanilla,F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature 402, 809 –813 (1999).
Hayden,F. G., Rollins,B. S. & Hay,A. J. Anti-influenza virus activity of the compound LY253963. Antiviral Res. 14, 25– 38 (1990).
Krug,R. M., Alonso-Caplen,F. V., Julkunen, I. & Katze,M. G. in The Influenza Virus 89–152 (Plenum, New York, 1989).
Schubert,U. et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770 –774 (2000).
Ortmann,B. et al. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science 277, 1306–1309 (1997).
Stam,N. J., Spits,H. & Ploegh,H. L. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol. 137, 2299– 2306 (1986).
Reits,E. A. J., Benham,A. M., Plougastel, B., Neefjes,J. & Trowsdale,J. Dynamics of proteasome distribution in living cells. EMBO J. 16, 6087– 6094 (1997).
Axelrod,D., Koppel,D. E., Schlessinger, J., Elson,E. & Webb,W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Jovin,T. M. & Vaz,W. L. Rotational and translational diffusion in membranes measured by fluorescence and phosphorescence methods. Methods Enzymol. 172, 471–513 (1989).
Acknowledgements
We thank K. Döring, M. van Ham and K. Jalink for discussions, L. Oomen for help with the confocal microscope, R. Wubbolts for support with microinjection, P. Vlottes (ICN Holland) for a sample of ribavirin, and A. Benham and T. Schumacher for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reits, E., Vos, J., Grommé, M. et al. The major substrates for TAP in vivo are derived from newly synthesized proteins. Nature 404, 774–778 (2000). https://doi.org/10.1038/35008103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35008103
This article is cited by
-
A guide to antigen processing and presentation
Nature Reviews Immunology (2022)
-
Deubiquitinating enzymes and the proteasome regulate preferential sets of ubiquitin substrates
Nature Communications (2022)
-
Machinery that guides immunity
Nature (2017)
-
Elimination of a signal sequence-uncleaved form of defective HLA protein through BAG6
Scientific Reports (2017)
-
Therapeutic targeting of naturally presented myeloperoxidase-derived HLA peptide ligands on myeloid leukemia cells by TCR-transgenic T cells
Leukemia (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.