Abstract
The Pax5 gene encoding the B-cell-specific activator protein (BSAP) is expressed within the haematopoietic system exclusively in the B-lymphoid lineage, where it is required in vivo for progression beyond the pro-B-cell stage. However, Pax5 is not essential for in vitro propagation of pro-B cells in the presence of interleukin-7 and stromal cells. Here we show that pro-B cells lacking Pax5 are also incapable of in vitro B-cell differentiation unless Pax5 expression is restored by retroviral transduction. Pax5-/- pro-B cells are not restricted in their lineage fate, as stimulation with appropriate cytokines induces them to differentiate into functional macrophages, osteoclasts, dendritic cells, granulocytes and natural killer cells. As expected for a clonogenic haematopoietic progenitor with lymphomyeloid developmental potential, the Pax5-/- pro-B cell expresses genes of different lineage-affiliated programmes, and restoration of Pax5 activity represses this lineage-promiscuous transcription. Pax5 therefore plays an essential role in B-lineage commitment by suppressing alternative lineage choices.
Similar content being viewed by others
Main
Originally published as Nature 397, 263–266; 1999
All types of blood cell are generated from a pluripotent haematopoietic stem cell (HSC) through developmentally restricted progenitors which undergo lineage commitment and subsequent differentiation along a single pathway. The lymphoid lineages develop through a common lymphoid progenitor (CLP) which gives rise to natural killer, B and T cells1. Early B-cell development can be dissected into different stages according to the rearrangement status of the immunoglobulin heavy-chain (IgH) locus, the expression of stage-specific cell-surface markers and growth factor requirements2,3. The earliest B-lineage precursor cells carry the IgH locus still in germline configuration. DH-JH recombination is subsequently initiated in pre-BI cells3, which are also known as early pro-B cells (fraction B)2. These pro-B cells can be cultured in vitro on stromal cells in the presence of interleukin-7 (IL-7) and express the B-cell surface proteins λ5, VpreB, Igα and Igβ (refs 3, 4). Completion of a functional VH-DJH rearrangement results in the expression of the pre-B-cell receptor and subsequent differentiation to small pre-B cells, which are no longer responsive to pro-B-cell growth conditions5.
The initiation of B-cell development critically depends on two transcription factors; the basic helix–loop–helix proteins encoded by the E2A gene and the early B-cell factor (EBF). In the absence of either protein, B-cell development is aborted at the earliest stage, before DH-JH rearrangement of the IgH gene6,7,8. Moreover, forced expression of E2A and EBF in haematopoietic precursor cells revealed that these regulators cooperatively induce the transcription of several B-lymphoid-specific genes9,10. Hence, loss- and gain-of-function experiments have implicated E2A and EBF in the control of B-lineage commitment.
A third transcriptional regulator involved in early B-lymphopoiesis is the B-cell-specific activator protein (BSAP), which is encoded by the Pax5 gene and recognizes DNA through the highly conserved paired domain characteristic of the Pax family of transcription factors11. Within the haematopoietic system, Pax5 is exclusively expressed in the B-lymphoid lineage from the earliest detectable precursor to the mature B-cell stage12,13. In the absence of Pax5, B-cell development is arrested in the fetal liver at a similarly early stage as in E2A and EBF mutant mice, whereas it proceeds in adult bone marrow up to the early pro-B (pre-BI) cell stage14,15. B-lymphopoiesis in the bone marrow is, however, not advanced by expression of a functionally rearranged µ-heavy-chain transgene5, indicating that the observed developmental block does not result from the inability of Pax5 -/- pro-B cells to undergo VH- DJH rearrangements at the IgH locus15. Pax5-/- pro-B cells can be cultured in vitro with similar efficiency as wild-type pro-B cells, a fact that has facilitated the genetic identification of Pax5 target genes15. BSAP (Pax5) functions both as a transcriptional activator and as a repressor, as it positively controls CD19, mb-1 (Igα), N-myc and Lef-1 expression and negatively regulates PD-1 transcription16. Apart from these target genes, Pax5-deficient and wild-type pro-B cells do not significantly differ in the expression of 50 B-cell-associated genes analysed16. This observation suggested that pro-B cells, even in the absence of Pax5, still undergo commitment to the B-lymphoid lineage. However, investigating the development potential of Pax5-/- pro-B cells, we now show that Pax5, instead of E2A and EBF, is the critical transcription factor involved in B-lineage commitment.
Pax5-/- pro-B cells lack B-lymphoid potential
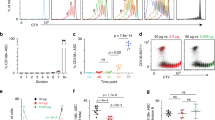
The RAG2 mutation also blocks B-cell development at the pro-B-cell stage in vivo17, like the Pax5 mutation15, but it is compatible with differentiation of these cells to mature B-lymphocytes in vitro18. We have therefore examined the in vitro differentiation potential of Pax5-/- pro-B cells by culturing them for three days without IL-7 and stromal (ST2) cells. To promote cell survival upon IL-7 withdrawal18, a retroviral bcl2 gene was introduced into Pax5-/- and wild-type pro-B cells. In the absence of IL-7 and ST2 cells, the wild-type cells complete the rearrangement of their immunoglobulin genes and differentiate to small CD40+IgM+ B cells18 ( Fig. 1). In contrast, Pax5-/- cells do not express any markers associated with late differentiation (Fig. 1; and data not shown). However, Pax5-/- pro-B cells infected with a Pax5-expressing retrovirus can differentiate into B cells in vitro (Fig. 1). Hence, the Pax5 mutation arrests B-cell development at the same early stage in vitro as in vivo, and this developmental block can be overcome by retrovirus-mediated expression of Pax5.
Wild-type and Pax5-/- pro-B cells expressing a retroviral bcl2 gene were cultured in the absence of stromal cells and IL-7 for three days in IMDM medium alone followed by flow cytometric analysis. Grating for CD19+ cells was used to select the Pax5-/- pro-B cells infected with a BSAP (Pax5)-expressing retrovirus16. FSC, forward scatter.
Pax5-/- pro-B cells are uncommitted
While growing pro-B-cell clones, we found that Pax5-/- pro-B cells, in contrast to wild-type pro-B cells, could survive for up to three weeks without medium change. Although the culture medium was depleted of IL-7 during this time, some Pax5-/- cells underwent a characteristic change in morphology and subsequently continued to proliferate. Whereas Pax5-/- pro-B cells grown in IL-7 are small and refractile, and contain little cytoplasm (Fig. 2a), these cells became larger and irregular in shape upon IL-7 depletion (Fig. 2b).
a, Confocal microscopic image of a Pax5-/- pro-B-cell colony after 10 days in IL-7 medium. b, Altered morphology of two representative colonies of the same pro-B-cell culture 30 days after the last medium change. c, Statistical analysis. Individual B220+ c-Kit+ pro-B cells, which were isolated by single-cell sorting directly from the bone marrow (ex vivo) or from established pro-B-cell cultures (in vitro) of the indicated genotypes, were grown on stromal ST2 cells in IL-7 medium, scored for colony formation at day 10 and subsequently examined for altered morphology at 3-day intervals (without medium change). The bcl2 gene was introduced by retroviral transduction. WT, wild-type.
To systematically investigate this phenomenon, we sorted individual pro-B cells into single wells of a 96-well plate, scored the growth of pro-B-cell colonies after 10 days in IL-7 medium and then monitored the frequency with which these colonies changed their morphology as IL-7 became limiting (Fig. 2c). For this purpose, pro-B cells were either freshly isolated from the bone marrow of Pax5-/-, RAG2-/- and wild-type mice (ex vivo) or were sorted from established pro-B cell cultures (in vitro). The Pax5-/- pro-B-cell clones changed their morphology at a high frequency (64–87%), irrespective of bcl2 expression, whereas the wild-type and RAG2-/- pro-B cells did not show this morphology change ( Fig. 2c). Our interpretation of these results is that the wild-type and RAG2-/- pro-B cells have undergone B-lineage commitment and can therefore only differentiate to non-proliferating B-cells upon IL-7 withdrawal18 (Fig. 1). However, under the same conditions Pax5-/- pro-B cells cannot develop along the B-lymphoid lineage (Fig. 1). Instead, they appear to differentiate along other haematopoietic pathways, indicating that these cells are not committed to the B-lymphoid lineage.
In vitro differentiation along monocytic lineages
The morphology of the cells shown in Fig. 2b was suggestive of myeloid cell types, in agreement with the fact that ST2 cells produce the cytokine macrophage colony-stimulating factor (M-CSF)19. We therefore assessed the potential of Pax5-/- pro-B cells to develop into macrophages by culturing them for 10–14 days on ST2 cells (in the absence of IL-7) followed by propagation in M-CSF (with or without ST2 cells). Cells differentiated in this manner displayed the characteristic morphology of enlarged vacuolar macrophages (Fig. 3a) and were positive for the macrophage markers Mac-1 and F4/80, but did not express the B-cell protein B220 and granulocyte marker Gr-1 ( Fig. 3c). The same cells efficiently phagocytosed fluorescently labelled Escherichia coli (Fig. 3b), indicating that Pax5-/- pro-B cells can differentiate into mature macrophages.
a, May–Grünwald–Giemsa staining of cytospin preparations of Pax5-/- pro-B cells cultured for 14 days on ST2 cells with or without IL-7. b, Phagocytosis. In vitro differentiated Pax5-/- macrophages were incubated with FITC-labelled E. coli. c, Flow cytometric analysis of macrophages derived from Pax5-/- pro-B cells. d, Confocal microscopic images of Pax5-/- pro-B cells grown for 16 days on ST2 cells with or without GM-CSF. e, MHC class II expression on cells differentiated in GM-CSF medium and stained with an anti-MHC class II antibody. f, Flow cytometric analysis of dendritic cells derived from Pax5-/- pro-B cells. The cells shown in c and f were grated on large size. g, Mixed leukocyte reaction. Increasing doses of γ-irradiated stimulator cells (cells per well) were incubated for four days with 2.5 × 105 responder cells isolated from lymph nodes of BALB/c mice. [3H]thymidine incorporation (c.p.m.) was measured during the last 14 h of incubation. Dendritic cells derived from two Pax5 -/- pro-B-cell lines (squares, diamonds) and splenocytes from allogeneic C57BL/6 (circles) or syngeneic BALB/c mice (triangles) were used as stimulator cells. h, Kinetic analysis of myeloid commitment. Bcl2-expressing pro-B cells deprived of IL-7 and ST2 cells were cultured with or without M-CSF. Viable cells were assessed for their ability to be recloned as pro-B cells in the presence of IL-7 and ST2 cells by limiting dilution analysis4. The re-cloning frequency is indicated relative to that of Pax5-/- pro-B cells at day 0. n.a., not applicable.
Using a cell-cloning assay, we next investigated the kinetics with which Pax5-/- pro-B cells become committed to the myeloid lineage. Bcl2-expressing wild-type and Pax5-/- pro-B cells were cultured in the absence of IL-7 and ST2 cells or in the presence of M-CSF for defined times. Subsequently, the proportion of viable cells that could be re-cloned as pro-B cells upon re-addition of stromal cells and IL-7 was determined by limiting dilution analysis. Wild-type pro-B cells rapidly lost their ability to be re-cloned (Fig. 3h) owing to efficient differentiation along the B-lymphoid lineage4. In contrast, the Pax5-/- pro-B cells, which are not yet committed to a lineage-specific programme, could be re-cloned even after 15 days of IL-7 and stromal cell withdrawal (Fig. 3h). M-CSF addition, however, reduced the cloning frequency of Pax5-/- pro-B cells 10-fold after six days and completely abolished it after 15 days (Fig. 3h). Hence, most cells initiated differentiation along the myeloid lineage and thus lost their IL-7 responsiveness within six days of M-CSF stimulation.
Dendritic cells also develop in the bone marrow from the same monocyte precursor as the macrophages, but require the cytokine granulocyte–macrophage CSF (GM-CSF) instead of M-CSF for terminal differentiation20,21. To generate dendritic cells from Pax5-/- pro-B cells, we applied a similar strategy as for macrophage development except that GM-CSF was added in the final differentiation step. After 16 days of GM-CSF stimulation, the cells were proliferating in small aggregates characteristic of dendritic cells20, whereas only macrophages grew in the absence of GM-CSF (Fig. 3d). Mature dendritic cells are antigen-presenting cells characterized by a stellate shape with multiple cytoplasmic projections and by high levels of MHC class II protein on their surfaces20,21. The Pax5-/- cells, differentiated in response to GM-CSF, exhibited the characteristic cell morphology and MHC class II expression (Fig. 3e) as well as the expected cell-surface phenotype (MHC-IIhigh CD11c+ Mac-1+ CD40+ CD8- B220-) of mature dendritic cells (Fig. 3f; and data not shown). These cells were further identified as dendritic cells by their ability to stimulate T-cell proliferation in a mixed leukocyte reaction (Fig. 3g). In this assay, allogeneic splenocytes of C57BL/6 mice elicit a proliferative response in T cells of BALB/c mice, in contrast to syngeneic splenocytes (Fig. 3g). Importantly, the Pax5-/- dendritic cells activated the T cells with a 10–30-fold higher efficiency then allogeneic splenocytes (Fig. 3g), in agreement with the fact that dendritic cells are significantly more potent than splenocytes in stimulating T-cell proliferation21. Pax5-/- pro-B cells can therefore also differentiate to become functional dendritic cells.
Osteoclasts constitute a third lineage which originates from a common monocyte precursor. Differentiation to bone-resorbing osteoclasts is controlled by the cytokine TRANCE (also known as OPGL, ODF or RANKL), which is presented on activated osteoblasts22. Following IL-7 withdrawal, we therefore cultured Pax5-/- pro-B cells on stromal ST2 cells which ectopically expressed TRANCE. Under these conditions, the pro-B cells differentiated within two weeks into cells expressing the osteoclast-specific enzyme tartrate-resistant acid phosphatase (TRAP; Fig. 4a). These differentiated cells had the multinucleated morphology of mature osteoclasts ( Fig. 4b) and formed resorption lacunae on artificial bone substrates (Fig. 4c). Hence, Pax5-/- pro-B cells can also develop into functional osteoclasts.
a, TRAP staining (red) of Pax5-/- cells cultured for two weeks on TRANCE-expressing ST2 cells. b, Multinucleated morphology of TRAP+ cells. Differentiated Pax5-/- cells were stained for TRAP activity (red) followed by counterstaining with haematoxylin (revealing the nuclei in blue) and cytospin centrifugation. c, Bone resorption. Osteoclasts derived from Pax5-/- pro-B cells were incubated on an osteologic bone disc with ST2-TRANCE cells. A multinucleated osteoclast (OC) is shown with the pits (P) resulting from its bone-resorbing activity. d–f, Cytospin preparations of May–Grünwald–Giemsa-stained cells. Pax5-/- pro-B cells initially grown in IL-7 medium (d) were cultured in the presence of IL-3, IL-6 and SCF for three weeks (e) and subsequently in G-CSF medium alone for six days (f). Granulocytes were identified by their segmented nuclear morphology (arrowhead in e).
Differentiation into granulocytes
Myeloid progenitors also give rise to cell types of the neutrophil lineage which characteristically contain a highly segmented nucleus and differentiate under the influence of the cytokines IL-6 and granulocyte CSF (G-CSF)23. To test the granulocytic potential of Pax5-/- pro-B cells, we cultured these cells for three weeks with the multilineage cytokines IL-3, IL-6 and stem-cell factor (SCF). Under these conditions, a small percentage of the Pax5-/- cells differentiated into granulocytic cells, as shown by their segmented nuclear morphology (Fig. 4e). Homogeneous differentiation to granulocytes was achieved by replacing the multilineage cytokines with G-CSF for six days (Fig. 4f). Pax5-/- pro-B cells can, therefore, also differentiate along the granulocytic lineage.
Differentiation into natural killer cells
The myeloid differentiation experiments unequivocally show that Pax5 -/- pro-B cells are not committed to the B-lymphoid lineage. Hence, the question arose of whether these cells also have properties of the recently described CLP and could thus give rise to natural killer (NK) cells1. NK-cell development critically depends on IL-15 (ref. 24), which can, at least in vitro, be substituted by IL-2 (ref. 25). To assess their NK-cell potential, the Pax5-/- pro-B cells were therefore cultured with IL-2 and stromal cells. Within 10 days, the Pax5-/- cells switched off expression of the B-cell protein B220, initiated synthesis of the NK-cell markers NK1.1, Ly49C and 2B4 and were identified as NK cells by their cell-surface phenotype (NK1.1+ Ly49C+ 2B4+IL-2RαlowIL-2RβlowMac-1low CD3ε-CD4-CD8-B220-; Fig. 5a and data not shown).
a, Flow cytometric analysis of Pax5-/- pro-B cells cultured for nine days with IL-2 and ST2 cells. b, Cytoxicity. Cells of two Pax5-/- pro-B-cell lines (closed and open symbols) were differentiated in IL-2 for nine days and assayed for their cytotoxicity against YAC-1 cells and LPS-treated splenocytes from wild-type C57BL/6 or β2m-/- mice27. Cytolytic assays were performed at the indicated effector to target cell ratios.
NK cells contribute to innate immunity by killing host cells that fail to express MHC class I proteins on their surfaces as a consequence of viral infection or tumorigenesis26. We therefore measured the cytolytic activity of in vitro differentiated Pax5-/- cells in a 51Cr release assay, using the sensitive YAC-1 tumour cells or LPS-stimulated lymphoblasts from β2-microglobulin-deficient (β2m-/-) mice, which lack MHC class I cell-surface expression27. Both target cells were lysed in a dose-dependent manner by NK cells derived from Pax5-/- pro-B cells, whereas LPS-activated target cells from a syngeneic C57BL/6 mouse were resistant to lysis (Fig. 5b). Hence, Pax5-/- pro-B cells can differentiate into functional NK cells.
Under all in vitro differentiation conditions analysed, we never detected Pax5-/- cells that expressed surface antigens characteristic of the T-lymphoid lineage, in agreement with the fact that the complex inductive microenvironment of the thymus is required for early T-cell development. When injected into RAG2-/- mice, Pax5-deficient pro-B cells were, however, able to fully reconstitute T-cell development in vivo28. Therefore, Pax5-/- pro-B cells can also give rise to lymphocytes except for B cells.
In vivo myeloid development of Pax5-/- pro-B cells
The observation that injected Pax5-/- pro-B cells home to the bone marrow and undergo self-renewal in RAG2-/- mice28 raises the question of whether these cells can also differentiate in vivo into myeloid cells, in addition to T-lymphocytes. However, we could not detect the formation of myeloid cell types in reconstituted RAG2-/- mice28, suggesting that the Pax5 -/- pro-B cells cannot efficiently compete with endogenous myeloid progenitors. For this reason, we analysed the in vivo myeloid potential of Pax5-/- pro-B cells in the c-fos-/- mouse, which has a developmental defect in the osteoclast lineage29,30. Consequently, c-fos-/- mice develop osteopetrotic bones and, owing to the absence of a bone marrow cavity, exhibit extramedullary haematopoiesis in the spleen30. As transplantation of Pax5-/- pro-B cells alone cannot rescue lethally irradiated mice (data not shown), we performed competitive reconstitution experiments by injecting Pax5-/- pro-B cells together with c-fos-/- splenocytes into lethally irradiated, newborn c-fos-/- mice. Four weeks after cell transfer, osteoclasts derived from Pax5-/- pro-B cells could be detected by TRAP staining on the surface of the calvariae of three reconstituted c-fos-/- mice (Fig. 6c), as in wild-type mice (Fig. 6a), whereas calvarial osteoclasts were never found in untreated c-fos-/- mice ( Fig. 6b). However, the reconstituted c-fos-/- mice showed neither tooth eruption nor development of a bone marrow cavity in the long bones (Fig. 6f). A few clusters of TRAP+ osteoclasts were nevertheless observed in the osteopetrotic bones of the reconstituted c-fos-/- mice ( Fig. 6f), in contrast to untreated c-fos-/- mice (Fig. 6e). Hence, the injected Pax5-/- pro-B cells gave rise to only a partial rescue of the c-fos mutant phenotype, possible owing to still inefficient competition with myeloid progenitors derived from the c-fos-/- HSC. Nevertheless, these experiments show that Pax5-/- pro-B cells can differentiate into at least one myeloid cell type in vivo.
Identification of osteoclasts in the calvariae (a–c) and long bones (d–f) of 4-week-old mice. TRAP staining (red) was used to detect osteoclasts on the calvarial surface and in sections of the tibia of wild-type (a, b), c-fos-/- (b, e) and reconstituted c-fos-/- ( c, f) mice. For reconstitution, 107 Pax5-/- pro-B cells and 107 splenocytes, isolated from an adult c-fos-/- mouse, were co-injected intraperitoneally into lethally irradiated c-fos-/- mice one day after birth. Four weeks later, osteoclast formation was analysed by TRAP histochemistry. BM, bone marrow cavity; GP, growth plate.
The multilineage potential is clonal
During this study, we tested the myeloid and lymphoid differentiation potential of five independently derived Pax5-/- pro-B-cell pools, of which three were infected with a bcl2-expressing retrovirus. All five pro-B-cell lines developed into macrophages in vitro and T cells in vivo, indicating that the ability to differentiate along the myeloid and lymphoid lineages is an intrinsic property of all Pax5-/- pro-B cells, irrespective of bcl2 expression. However, it could be argued that, despite their growth in IL-7, the Pax5-/- pro-B-cell pools may correspond to a heterogeneous mixture of lineage-committed progenitors. To exclude this possibility, we have analysed the differentiation potential of eight subclones which were generated by single-cell sorting of Pax5-/- pro-B-cell pools. In the appropriate environment, all eight subclones efficiently developed into macrophages, osteoclasts, dendritic cells (in vitro) and T cells (in vivo). The differentiation into granulocytes and NK cells was variable, possibly indicating either that our in vitro differentiation conditions were not optimal for these two lineages or that in vitro cultured Pax5-/- pro-B cells gradually lose the capacity to generate these two cell types. Nevertheless, our results unequivocally show that Pax5-/- pro-B cells are multipotent haematopoietic progenitors which maintain their differentiation potential after single-cell cloning.
Pax5 represses lineage-promiscuous transcription
Multipotent haematopoietic progenitors simultaneously express genes from different lineage-affiliated programs, in a process known as ‘multilineage priming’31. Consistent with its progenitor status, the Pax5-/- pro-B cell is therefore expected to exhibit lineage-promiscuous gene expression, even though Pax5-/- pro-B cells differ only minimally from committed wild-type pro-B cells in the expression of 50 B-cell-associated genes analysed16. To investigate this issue, we used the polymerase chain reaction with reverse transcription (RT-PCR) to compare the expression of different lineage-specific genes between uncommitted Pax5-/- pro-B-cell lines and committed pro-B cells derived either by retrovirus-mediated reconstitution of Pax5 expression or by isolation from wild-type and RAG2-/- bone marrow (Fig. 7a, b). According to their expression pattern, the different genes could be grouped into three categories (Fig. 7; and data not shown). The transcripts of several genes, including CD3ε (T-lymphoid), G-CSF-Rα (granulocytic) and βmaj–globin (erythroid), were not detected in any pro-B-cell line, whereas they could be readily amplified from control tissues. Other genes were equally expressed in pro-B cells of all genotypes. This category comprises the genes B29 (B-lymphoid), GM-CSF-Rα (myeloid), EPO-R (erythroid), Tcf-1 (T-lymphoid) and GATA-3 (T-lymphoid), although the last two transcription factor genes were expressed only at a very low level. Some genes, on the other hand, were exclusively expressed in Pax5-/- pro-B cells, but not in any of the committed pro-B cells. This group includes the relatively abundant transcripts of the myeloid M-CSF receptor (M-CSF-R; c-fms) and myeloperoxidase (MPO) genes as well as the rare messenger RNAs of the GATA-1 (erythroid), perforin (NK cell) and pTα (T lymphoid) genes. Importantly, the lineage-promiscuous expression of these genes was efficiently repressed in Pax5-/- pro-B cells by restoring Pax5 activity using retroviral transduction (Fig. 7a, b).
a, b, RT-PCR analysis. The following pro-B cells grown on stromal ST2 cells in the presence of IL-7 were analysed: four independent Pax5-/- pro-B cell lines, Pax5-/- pro-B cells reconstituted with a Pax5-expressing retrovirus (KO-Pax5) and pro-B cells established from wild-type (+/+) and RAG2-/- bone marrow. The PCR products were visualized on agarose gels by ethidium bromide staining (a) or Southern blot analysis (b). The quantity of input cDNA was normalized by analysing the control HPRT transcript. No reverse transcriptase was added in lane - RT. c, Loss of Pax5 expression upon myeloid differentiation. Pax5-/- pro-B cells and wild-type splenocytes were cultured for 10 days with M-CSF and ST2 cells. Pax5 expression was subsequently monitored by RT-PCR with primers from Pax5 exon 1A and lacZ sequences inserted in the targeted Pax5 locus14. Pax5-/- pro-B cells grown in IL-7 medium (KO + IL-7) were used as positive control.
We next investigated whether the transcription of B-cell-specific genes such as B29 (Igβ) and Pax5 is repressed in Pax5 -/- pro-B cells upon entry into the myeloid lineage. Ten days after replacement of IL-7 with M-CSF, four independent Pax5-/- cell lines efficiently downregulated the expression of B29 as well as of the lacZ gene inserted in the targeted Pax5 locus (Fig. 7c). In summary, our data indicate that an essential fucntion of Pax5 in B-lineage commitment is to repress the lineage-promiscuous transcription of non-B-lymphoid genes. Conversely, transcription of the Pax5 gene is itself inactivated upon differentiation to other haematopoietic lineages.
Discussion
Differentiation of the HSC into distinct blood cell types is thought to progress through intermediate progenitor cells with restricted developmental potential. This view of haematopoiesis is challenged by our finding that the Pax5-/- pro-B cell possesses, at least under in vitro conditions, a broad developmental potential similar to that of the HSC itself. So far, however, we have been unable to differentiate the Pax5 -/- pro-B cell along the erythroid and megakaryocytic lineages, which may reflect a more restricted developmental capacity of this cell or our failure to define appropriate differentiation conditions for these lineages. Moreover, Pax5-/- pro-B cells have so far failed to reconstitute the entire haematopoietic system in transplantation experiments28. In the absence of this characteristic property of the HSC, the Pax5-/- pro-B cell must be classified as a haematopoietic progenitor cell with broad lymphoid and myeloid differentiation potential.
A close developmental connection between the lymphoid and myeloid lineages has been demonstrated by the identification of a B-cell/macrophage-restricted precursor, on the basis of several criteria32. First, certain human acute leukaemias co-express B-cell and macrophage characteristics, indicating that they may be derived from a transformed progenitor cell of both lineages33. Second, mice expressing the Eµ-myc transgene generate immature lymphomas that can differentiate along the B-lymphoid and macrophage lineages34,35. Third, several immortalized pre-B-cell lines can be converted into macrophages36,37,38. Finally, the murine fetal liver contains a small subpopulation of clonogenic B-cell/macrophage progenitors39. Fetal liver cells with an expanded lymphomyeloid potential have been identified by several groups by varying the conditions of their in vitro differentiation assays40,41,42. A progenitor cell with a similar developmental capacity has so far not been isolated from mouse bone marrow1, whereas human bone marrow contains cells with the potential to differentiate along the T, B, NK and dendritic cell lineages43. The interpretation of these studies is, however, complicated by the fact that the isolated progenitor cells are rare, correspond to only a transition stage in haematopoietic development and cannot be expanded long-term in vitro in an uncommitted state, thus precluding any detailed analysis. In contrast, the Pax5-/- pro-B cell can be propagated in vitro as an uncommitted haematopoietic progenitor. Moreover, this cell originates from the bone marrow and, except for B-cell development, exhibits the full spectrum of myeloid and lymphoid differentiation.
The development of lymphocytes, but not of myeloid cells, is critically dependent on IL-7, which promotes the proliferation and/or survival of lymphoid precursors44. Expression of the IL-7 receptor was used as a criterion for isolating the CLP from mouse bone marrow1. This CLP has the capacity to rapidly reconstitute B, T and NK cells in vivo , but lacks myeloid differentiation potential in vivo and in vitro1. A close relationship between the CLP and Pax5 -/- pro-B cell is suggested by the fact that the Pax5 -/- pro-B cell also expresses the IL-7 receptor15, depends on IL-7 for in vitro propagation15 and possesses lymphoid developmental potential. However, in contrast to the CLP, the Pax5-/- pro-B cell can differentiate along myeloid lineages in vitro and generate osteoclasts in vivo after injection into c-fos-/- mice. Given the transitory nature of the CLP, it is conceivable that, shortly after its isolation, the CLP may express Pax5 or an equivalent commitment factor of the NK- and T-cell lineages, which subsequently prevents differentiation along myeloid lineages. In this view, the full developmental capacity of the CLP is only revealed under conditions that interfere with commitment to the lymphoid lineages, such as IL-7-dependent proliferation in the absence of Pax5 function.
Multilineage priming of gene expression is a characteristic property of early haematopoietic progenitor cells45. This process ensures that genes of different haematopoietic lineages are co-expressed within a single cell, albeit often at a low level31. The lineage-promiscuous gene expression observed in the Pax5-/- pro-B cell thus provides molecular evidence for the progenitor status of this cell. The expression of M-CSF-R, GM-CSF-Rα and Epo-R may also explain why the Pax5-/- pro-B cell can respond to different cytokines. For instance, expression of the M-CSF receptor renders the Pax5-/- pro-B cell responsive to M-CSF, which is produced by the ST2 feeder cells used for pro-B-cell culture19. IL-7 is, however, dominant over M-CSF in lymphoid precursor cells38. As a consequence, M-CSF can support monocytic differentiation and proliferation only at limiting IL-7 concentrations, which explains the observed morphology change of Pax5-/- pro-B cells in IL-7-depleted medium.
Early events in lineage specification involve the control of proliferation, survival and commitment of lineage-restricted precursor cells. The loss of an entire lineage by gene targeting can therefore result from interference with any one of these processes and does not necessarily imply a role of the mutated gene in lineage commitment. A hallmark of commitment is the stabilization of a lineage-specific gene-expression programme which permanently inhibits alternative lineage choices45. Consequently, restriction of developmental plasticity provides a better criterion for defining lineage commitment. Loss-of-function experiments can therefore implicate a transcription factor in lineage commitment only if the lack of ‘forward’ differentiation is accompanied by maintenance of developmental plasticity in the transcription-factor-deficient progenitor cell. However, it must be possible to grow a mutant progenitor in vitro before its developmental potential can be analysed. So far, Pax5 is the only lineage-specific transcription factor to fulfil this criterion, as Pax5-/- pro-B cells can differentiate along multiple myeloid and lymphoid lineages with the exception of the B-cell pathway. Restoration of Pax5 activity overcomes the B-cell developmental block, thus identifying Pax5 as the critical B-lineage commitment factor. Surprisingly, considerable progression down the B-cell pathway is possible in the absence of commitment, as Pax5-/- pro-B cells express many genes previously considered to be indicative of B-lineage commitment15,16. These genes include Igα(mb-1), Igβ(B29), VpreB and λ5 as well as the sterile transcripts of the IgH locus, all of which are under single or combinatorial control by the transcription factors E2A and EBF7,8,9,46. Consistently, both E2A and EBF, which act upstream of Pax5 in the genetic hierarchy of B-cell development7,8, are equally expressed in Pax5-/- and wild-type pro-B cells15. Hence, the Pax5 mutation dissociates the initial activation of B-lineage-specific gene expression by E2A and EBF from the Pax5-dependent control of B-lineage commitment. Pax5 fulfils a dual role in B-lineage commitment, as it activates the expression of B-cell-specific genes and simultaneously represses the lineage-promiscuous transcription of other haematopoietic genes. The transcriptional activation is best exemplified by CD19, whose expression is strictly dependent on Pax5 (ref. 16) and should thus be regarded as a decisive marker of B-lineage commitment. Moreover, the Pax5-dependent repression of the M-CSF-R gene illustrates at the molecular level how the developmental potential is restricted at commitment by rendering B-cell precursors unresponsive to lineage-inappropriate cytokines such as M-CSF.
A long-standing debate concerns whether lineage commitment in the haematopoietic system occurs in a cell-autonomous, stochastic manner or is controlled by instructive signals from the local environment47,48. Much attention has been paid to the role of haematopoietic growth factors in this process, but no evidence for any defect in lineage commitment has been observed despite the analysis of many growth factor gene knock-outs48. Instead, growth factors seem to perform a selective role by promoting the survival and/or proliferation of independently committed cells. We have shown that the expression of Pax5 is randomly initiated from only one of its two alleles at the onset of B-lymphopoiesis49. The transcription of a single allele indicates that the lineage commitment gene Pax5 is activated in a relatively inefficient, stochastic manner in the multipotent progenitor49. However, once this stochastic event has occurred, it restricts the various developmental options of the progenitor to the B-cell pathway. Hence, our data support the notion that B-lineage commitment is a stochastic rather than a deterministic process.
Methods
Mice
Pax5-/-, RAG2-/- nd c-fos-/- mice14,17,29 were maintained on the C57BL/6x129/Sv background and genotyped as described5,15,29.
Pro-B cell culture
Iscove's modified Dulbecco's medium supplemented with 50 µM β-mercaptoethanol, 1 mM glutamine, 2% heat-inactivated fetal calf serum and 0.03% (w/v) primatone was used for all cell-culture experiments (referred to as IMDM medium). Pro-B cells isolated from bone marrow of 2-week-old mice were cultured on γ-irradiated ST2 cells in IMDM medium containing IL-7 (ref. 15).
Retroviral infection
The retrovirus pBabe–BSAP has been described16; pBabe–TRANCE was provided by K. Matsuo and E. F. Wagner. Murine bcl2 cDNA was inserted into the histidinol resistance vector pMV-10 to generate a bcl2-expressing retrovirus. Transfected GP + E-86 packaging cells were selected with 2.5 µg ml-1 puromycin or 10 mM histidinol (Sigma). Pro-B cells were infected by coculture with puromycin-histidinol-resistant ST2 cells and virus-producing packaging cells followed by puromycin (2.5 µg ml-1) or histidinol (1 mM) selection.
Antibodies
Anti-CD40 (FGK45.5), anti-µH (M41), anti-CD19 (1D3), anti-c-Kit (ACK4) and anti-MHC class II (M5-114) antibodies were purified and biotinylated as described15. Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labelled anti-B220 (RA3-6B2), FITC-labelled anti-Ly49C (5E6) and anti-Mac-1 (M1/70), PE-conjugated CD11c (HL3) and Gr-1 (RB6-8C5), biotinylated anti-CD23 (B3B4) and anti-NK1.1 (PK136) antibodies and allophycocyanin (APC)-conjugated streptavidin were obtained from PharMingen; PE-conjugated anti-F4/80 (C1:A3-1) antibody from Serotec; and PE-labelled streptavidin from Southern Biotechnology Associates.
Flow cytometry
Cells were stained and analysed on a FACSCalibur (Becton Dickinson) as described14. Biotinylated antibodies were revealed with PE- or APC-streptavidin. Myeloid cells were incubated with 10% heat-inactivated rat serum before antibody staining.
Growth factors
Murine M-CSF (used at a final concentration of 25 ng ml-1), G-CSF (25 ng ml-1) and SCF (100 ng ml-1) were purchased from R&D Systems. All other cytokines were produced by X63 or J558L cell transfectants50 and used at a concentration of 1% (IL-7), 2% (IL-3, IL-6), 5% (IL-2, IL-4) and 10% (GM-CSF) conditioned supernatant.
Cell morphology assay
Individual B220+c-Kit+ pro-B cells from bone marrow (ex vivo) or long-term pro-B-cell cultures (in vitro) were sorted by a FACSVantage TSO flow cytometer (Becton Dickinson) into single wells of 96-well plates containing ST2 cells in IL-7 medium. Colonies of small, round, refractile pro-B cells were identified after 10 days and thereafter scored for morphology changes defined by an increase in cell size, elongation and acquisition of granular vacuolated cytoplasm.
Cell-cloning assay
The cloning frequency of pro-B cells cultured on ST2 cells plus IL-7, in medium alone or in the presence of M-CSF, was assessed by limiting dilution analysis as described4. Cell numbers were normalized for viable cells (determined by trypan blue exclusion) before dilution into 96-well plates.
In vitro differentiation
All differentiation assays were performed with in vitro cultured pro-B cells. B lymphocytes: pro-B cells were incubated at 1–3 × 106 cells per ml for three days in IMDM medium alone as described18. NK cells: pro-B cells were differentiated for 10 days in the presence of IL-2 and ST2 cells. Cytoxicity assays were performed with YAC-1 cells or LPS-stimulated splenocytes as described25. Macrophages: Pax5-/- pro-B cells were differentiated in IMDM medium with ST2 cells for 10–14 days followed by terminal differentiation in M-CSF medium for seven days. Cells were transferred to chamber slides (Nalge Nunc), incubated with 107 FITC-labelled heat-inactivated E. coli (Molecular Probes) for 1–2 h at 37 °C, washed twice in PBS, fixed in 2% paraformaldehyde for 10 min, air-dried, mounted in VectaShield (Vector Laboratories) and DABCO (10 µg µl-1, Sigma)–DAPI (0.15 µg µl-1, Sigma) solutions (1:1) and analysed on a fluorescence microscope (Zeiss Axiophot) with a CCD camera (Photometrics). Dendritic cells: Pax5 -/- pro-B cells were differentiated for 16 days on ST2 cells in GM-CSF medium. For immunocytochemical analysis, cells were flushed off the stromal cell layer and stained with PE-anti-MHC class II antibodies before cytospin centrifugation and fluorescence microscopy. Differentiated cells were tested for antigen presentation by the mixed leukocyte reaction as described20. Osteoclasts: Pax5-/- pro-B cells were cultured for two weeks with M-CSF and ST2 cells expressing TRANCE. Cells were fixed in 3.7% formaldehyde for 30 min, dried and subjected to TRAP staining (Sigma). At day 10 of differentiation, cells were transferred onto osteologic bone discs (Millenium Biologic) together with ST2–TRANCE cells in M-CSF medium containing 100 nM dexamethasone, 10 mM vitamin D3 and 50 µg ml-1 vitamin C. Bone resorption was analysed after an additional 10-day incubation. Granulocytes: Pax5-/- pro-B cells were cultured for three weeks in IMDM medium containing IL-3, IL-6 and SCF and then incubated for six days in IMDM medium containing G-CSF alone. The cell morphology was analysed by May–Grünwald–Giesma staining.
In vivo osteoclast development
Newborn mice were lethally irradiated (800 rad) and then intraperitoneally injected with a mixture of 107 in vitro cultured Pax5 -/- pro-B cells and 107 splenocytes from an adult c-fos-/- mouse. Four weeks later, osteoclast formation was analysed by TRAP staining of isolated calvariae and histological sections of long bones30.
RT-PCR
Total RNA (2 µg) from pro-B cells and lymphoid tissues was reverse-transcribed and PCR-amplified as described49. The following primers were used: Pax5–lacZ, 5′-CATGGCGAGAAGCTCTTTAGTTCC-3′ and 5′-TGCAAGGCGATTAAGTTGGGTAAC-3′, CD3ε, 5′-GTCTCCATCTCAGGAACCAGT-3′ and 5′-ATAGTCTGGGTTGGGAACAGG-3′. All other primers have been described15,25,28,31. PCR products were identified on agarose gels by ethidium bromide staining or Southern hybridization.
References
Kondo,M., Weissman,I. L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661– 672 (1997).
Hardy,R. R., Carmack,C. E., Shinton,S. A., Kemp,J. D. & Kayakawa,K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 (1991).
Rolink,A., Grawunder,U., Winkler,T. H., Karasuyama,H. & Melchers,F. IL-2 receptor α chain (CD25,TAC) expression defines a crucial stage in pre-B cell development. Int. Immunol. 6, 1257–1264 (1994).
Rolink,A., Kudo,A., Karasuyama,H., Kikuchi,Y. & Melchers, F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen rective B cells in vitro and in vivo. EMBO J. 10, 327–336 (1991).
Thévenin,C., Nutt,S. L. & Busslinger, M. Early function of Pax5 (BSAP) prior to the pre-B cell receptor stage of B lymphopoiesis. J. Exp. Med. 188 , 735–744 (1998).
Zhuang,Y., Soriano,P. & Weintraub, H. The helix-loop-helix gene E2A is required for B cell formation. Cell 79, 875– 884 (1994).
Bain,G. et al. E2A proteins are required for proper B cell development and initiation of immunoglobulin gene rearrangements. Cell 79, 885–892 (1994).
Lin,H. & Grosschedl,R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature 376, 263–267 (1995).
Sigvardsson,M., O'Riordan,M. & Grosschedl, R. EBF and E47 collaborate to induce expression of the endogenous immunoglobulin surrogate light chain genes. Immunity 7, 25–36 (1997 ).
Kee,B. L. & Murre,C. Induction of early B cell factor (EBF) and multiple B lineage genes by the basic helix-loop-helix transcription factor E12. J. Exp. Med. 188, 699– 713 (1998).
Busslinger,M. & Nutt,S. L. in Molecular Biology of B-Cell and T-Cell Development (eds Monroe, J. G. & Rothenberg, E. V.) 83–110 (Humana, Totowa, New Jersey, 1998).
Adams,B. et al. Pax-5 encodes the transcription factor BSAP and is expressed in B lymphocytes, the developing CNS, and adult testis. Genes Dev. 6, 1589–1607 ( 1992).
Li, Y.-S., Wasserman,R., Hayakawa,K. & Hardy,R. R. Identification of the earliest B lineage stage in mouse bone marrow. Immunity 5, 527–535 ( 1996).
Urbánek,P., Wang, Z.-Q., Fetka,I., Wagner,E. F. & Busslinger, M. Complete block of early B cell differentiation and altered patterning of the posterior midbrain in mice lacking Pax5/BSAP. Cell 79, 901–912 ( 1994).
Nutt,S. L., Urbánek,P., Rolink, A. & Busslinger,M. Essential functions of Pax5 (BSAP) in pro-B cell development: difference between fetal and adult B lymphopoiesis and reduced V-to-DJ recombination at the IgH locus. Genes Dev. 11, 476–491 (1997).
Nutt,S. L., Morrison,A. M., Dörfler, P., Rolink,A. & Busslinger,M. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 17, 2319–2333 (1998).
Shinkai,Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Rolink,A., Melchers,F. & Andersson, J. The SCID but not the RAG-2 gene product is required for Sµ-Sε heavy chain class switching. Immunity 5, 319–330 (1996).
Yamane,T. et al. Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood 90, 3516–3523 ( 1997).
Inaba,K. et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176, 1693– 1702 (1992).
Branchereau,J. & Steinman,R. M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Kong, Y.-Y. et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397, 315 –323 (1999).
Liu,F., Poursine-Laurent,J., Wu,H. Y. & Link,D. C. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood 90, 2583–2590 (1997).
Ogasawara,K. et al. Requirement for IRF-1 in the microenvironment supporting development of natural killer cells. Nature 391, 700 –703 (1998).
Rolink,A. et al. A subpopulation of B220+ cells in murine bone marrow does not express CD19 and contains natural killer cell progenitors. J. Exp. Med. 183, 187– 194 (1996).
Raulet,D. H. Development and tolerance of natural killer cells. Curr. Opin. Immunol. 11, 129–134 ( 1999).
Liao, N.-S., Bix,M., Zijlstra,M., Jaenisch,R. & Raulet, D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253, 199–202 (1991).
Rolink,A. G., Nutt,S. L., Melchers,F. & Busslinger,M. Long-term in vivo reconstitution of T cell development by Pax5-deficient B cell progenitors. Nature 401, 603– 606 (1999).
Wang, Z.-Q. et al. Bone and haematopoietic defects in mice lacking c-fos. Nature 360, 741–745 ( 1992).
Grigoriadis,A. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443– 448 (1994).
Hu,M. et al. Multilineage gene expression precedes commitment in the hematopoietic system. Genes Dev. 11, 774– 785 (1997).
Borrello,M. A. & Phipps,R. P. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol. Today 17, 471– 475 (1996).
Akashi,K. et al. Simultaneous occurrence of myelomonocytic leukemia and multiple myeloma: involvement of common leukemic progenitors and their developmental abnormality of “lineage infidelity”. J. Cell Physiol. 148, 446–456 ( 1991).
Klinken,S. P., Alexander,W. S. & Adams, J. M. Hematopoietic lineage switch: v-raf oncogene converts Eµ-myc transgenic B cells into macrophages. Cell 53, 857–867 ( 1988).
Strasser,A., Elefanty,A. G., Harris,A. W. & Cory,S. Progenitor tumours from Eµ-bcl-2-myc transgenic mice have lymphomyeloid differentiation potential and reveal developmental differences in cells survival. EMBO J. 15, 3823–3824 (1996).
Davidson,W. F., Pierce,J. H., Rudikoff,S. & Morse III,H. C. Relationships between B cell and myeloid differentiation: studies with a B lymphocyte progenitor line, HAFTL-1. J. Exp. Med. 168 , 389–407 (1988).
Principato,M. et al. Transformation of murine bone marrow cells with combined v- raf-v-myc oncogenes yields clonally related mature B cells and macrophages. Mol. Cell. Biol. 10, 3562– 3568 (1990).
Borzillo,G. V., Ashmun,R. A. & Sherr, C. J. Macrophage lineage switching of murine early pre-B lymphoid cells expressing transduced fms genes. Mol. Cell. Biol. 10, 2703–2714 ( 1990).
Cumano,A., Paige,C. J., Iscove,N. N. & Brady,G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature 356, 612–615 ( 1992).
Kawamoto,H., Ohmura,K. & Katsura,Y. Direct evidence for the commitment of hematopoietic stem cells to T, B and myeloid lineages in murine fetal liver. Int. Immunol. 9, 1011–1019 ( 1997).
Aiba,Y. & Ogawa,M. Development of natural killer cells, B lymphocytes, macrophages, and mast cells from single hematopoietic progenitors in culture of murine fetal liver cells. Blood 90, 3923–3930 (1997).
Lacaud,G., Carlsson,L. & Keller, G. Identification of a fetal hematopoietic precursor with B cell, T cell, and macrophage potential. Immunity 9, 827–838 (1998).
Galy,A., Travis,M., Cen,D. & Chen,B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3, 459– 473 (1995).
van Freeden-Jeffry,U. et al. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526 (1995).
Enver,T. & Greaves,M. Loops, lineages, and leukemia. Cell 94, 9–12 ( 1998).
Åkerblad,P., Rosberg,M., Leanderson,T. & Sigvardsson,M. The B29 (immunoglobulin β-chain) gene is a genetic target for early B-cell factor. Mol. Cell. Biol. 19, 392–401 (1999).
Metcalf,D. Lineage commitment and maturation in hematopoietic cells: the case for extrinsic regulation. Blood 92, 345– 348 (1998).
Enver,T., Heyworth,C. M. & Dexter, T. M. Do stem cells play dice? Blood 92, 348–351 (1998).
Nutt,S. L. et al. Independent regulation of the two Pax5 alleles during B-cell development. Nature Genet. 21, 390 –395 (1999).
Karasuyama,H. & Melchers,F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 and 5, using modified cDNA expression vectors. Eur. J. Immunol. 18, 97–104 (1988).
Acknowledgements
We thank K. Matsuo and E. F. Wagner for c-fos-/- mice, ST2-TRANCE cells and advice concerning osteoclast biology; F. Alt for RAG2-/- mice; P. Steinlein and M. Dessing for FACS sorting and assistance with photography; and H. Beug for critical reading of the manuscript. This work was supported by the I.M.P. Vienna, by a grant from the Austrian Industrial Research Promotion Fund and by the Basel Institute for Immunology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nutt, S., Heavey, B., Rolink, A. et al. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature 402 (Suppl 6763), 14–20 (1999). https://doi.org/10.1038/35005514
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35005514
This article is cited by
-
Unusual nonlinear switching in branched carbon nanotube nanocomposites
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.