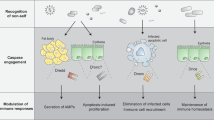
Abstract
The Drosophila immune response uses many of the same components as the mammalian innate immune response, including signalling pathways that activate transcription factors of the Rel/NK-κB family1,2,3,4. In response to infection, two Rel proteins, Dif and Dorsal, translocate from the cytoplasm to the nuclei of larval fat-body cells1,2,5. The Toll signalling pathway, which controls dorsal–ventral patterning during Drosophila embryogenesis6, regulates the nuclear import of Dorsal in the immune response2,7, but here we show that the Toll pathway is not required for nuclear import of Dif. Cytoplasmic retention of both Dorsal and Dif depends on Cactus protein; nuclear import of Dorsal and Dif is accompanied by degradation of Cactus. Therefore the two signalling pathways that target Cactus for degradation must discriminate between Cactus–Dorsal and Cactus–Dif complexes. We identified new genes that are required for normal induction of transcription of an antibacterial peptide during the immune response. Mutations in three of these genes prevent nuclear import of Dif in response to infection, and define new components of signalling pathways involving Rel. Mutations in three other genes cause constitutive nuclear localization of Dif; these mutations may block Rel protein activity by a novel mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ip, Y. T. et al. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell 75, 753–763 (1993).
Lemaitre, B. et al. Functional analysis and regulation of nuclear import of Dorsal during the immune response in Drosophila. EMBO J. 14, 536–545 (1995).
Dushay, M., Åsling, B. & Hultmark, D. Origins of immunity: Relish, a compound Rel-like gene in the antibacterial defense of Drosophila. Proc. Natl Acad. Sci. USA 93, 10343–10347 (1996).
Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A. Jr Ahuman homologue of Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 (1997).
Reichhart, J.-M. et al. Expression and nuclear translocation of the rel/NF-κB-related morphogen dorsal during the immune response of Drosophila. C. R. Acad. Sci. III 316, 1218–1224 (1993).
Belvin, M. P. & Anderson, K. V. Aconserved signaling pathway: The Drosophila Toll-Dorsal Pathway. Annu. Rev. Cell Dev. Biol. 12, 393–416 (1996).
Lemaitre, B., Nicolas, E., Michaut, L., Reichhardt, J.-M. & Hoffmann, J. A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 (1996).
Geisler, R., Bergmann, A., Hiromi, Y. & Nüsslein-Volhard, C. cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the IκB gene family of vertebrates. Cell 71, 613–621 (1992).
Kidd, S. Characterization of the Drosophila cactus locus and analysis of interactions between cactus and dorsal proteins. Cell 71, 623–635 (1992).
Roth, S., Stein, D. & Nüsslein-Volhard, C. Agradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell 59, 1189–1202 (1989).
Belvin, M. P., Jin, Y. & Anderson, K. V. Cactus protein degradation mediates Drosophila dorsal-ventral signaling. Genes Dev. 9, 783–793 (1995).
Bergmann, A. et al. Agradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech. Dev. 60, 109–123 (1996).
Reach, M. et al. Agradient of Cactus protein degradation establishes dorsoventral polarity in the Drosophila embryo. Dev. Biol. 180, 353–364 (1996).
DiDonato, J. A., Mercurio, F. & Karin, M. Phosphorylation of IκBα precedes but is not sufficient for its dissociation from NF-κB. Mol. Cell Biol. 15, 1302–1311 (1995).
Lin, Y. C., Brown, K. & Siebenlist, U. Activation of NF-κB requires proteolysis of the inhibitor IκBα: signal-induced phosphorylation of IκBα alone does not release active NF-κB. Proc. Natl Acad. Sci. USA 92, 552–556 (1995).
Miyamoto, S., Maki, M., Schmitt, M. J., Hatanaka, M. & Verma, I. M. Tumor necrosis factor α-induced phosphorylation of IκBα is a signal for its degradation but not dissociation from NF-κB. Proc. Natl Acad. Sci. USA 91, 12740–12744 (1994).
Hoffmann, J. A. & Reichhart, J.-M. Drosophila immunity. Trends Cell Biol. 7, 309–316 (1997).
Lemaitre, B. et al. Arecessive mutation, immune deficiency(imd), defines two distinct control pathways in the Drosophila host defense. Proc. Natl Acad. Sci. USA 92, 9465–9469 (1995).
Corbo, J. C. & Levine, M. Characterization of an immunodeficiency mutant in Drosophila. Mech. Dev. 55, 211–220 (1996).
Reichhart, J.-M. et al. Insect immunity: developmental and inducible activity of the Drosophila diptericin promoter. EMBO J. 11, 1469–1477 (1992).
Williams, M. J., Rodriguez, A., Kimbrell, D. A. & Eldon, E. D. The 18-wheeler mutation reveals complex antibacterial gene regulation in Drosophila host defense. EMBO J. 16, 6120–6130 (1997).
Hoffmann, J. A., Hetru, C. & Reichhart, J.-M. The humoral antibacterial response of Drosophila. FEBS Lett. 325, 63–66 (1993).
Kadalayil, L., Petersen, U.-M. & Engström, Y. Adjacent GATA and κB-like motifs regulate the expression of a Drosophila immune gene. Nucleic Acids Res. 25, 1233–1239 (1997).
Arenzana-Seisdedos, F. et al. Inducible nuclear expression of newly synthesized IκBα negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol. Cell Biol. 15, 2689–2696 (1995).
Beg, A. A., Sha, W. C., Bronson, R. T. & Baltimore, D. Constitutive NF-κB activation, enhanced granulopoiesis and neonatal lethality in IκBα-deficient mice. Genes Dev 9, 2736–2746 (1995).
Klement, J. F. et al. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol. Cell Biol. 16, 2341–2349 (1996).
Stancovski, I. & Baltimore, D. NF-κB activation: the IκB kinase revealed? Cell 91, 299–302 (1997).
Anderson, K. V. & Nüsslein-Volhard, C. Information for the dorsal-ventral pattern of the Drosophila embryo is stored as maternal mRNA. Nature 311, 223–227 (1984).
Acknowledgements
We thank R. Steward for antibodies to Dorsal and Cactus; Y. Engström for antibody against Dif; B. Lemaitre for the diptericin-lacZ transgenic flies; M. Dushay for the diptericin cDNA; Y. Lu and S. Li for help with mapping; and M. Baylies, M. Belvin and T. Bestor for comments on the manuscript. This work was supported by NIH and NSF grants to K.V.A. and the Memorial Sloan-Kettering Cancer Center Support Grant. L.P.W. is a Leukemia Society of America Special Fellow.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wu, L., Anderson, K. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 392, 93–97 (1998). https://doi.org/10.1038/32195
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/32195
This article is cited by
-
Toll signaling promotes JNK-dependent apoptosis in Drosophila
Cell Division (2020)
-
Brown marmorated stink bug, Halyomorpha halys (Stål), genome: putative underpinnings of polyphagy, insecticide resistance potential and biology of a top worldwide pest
BMC Genomics (2020)
-
TmCactin plays an important role in Gram-negative and -positive bacterial infection by regulating expression of 7 AMP genes in Tenebrio molitor
Scientific Reports (2017)
-
Differential modulation of the cellular and humoral immune responses in Drosophila is mediated by the endosomal ARF1-Asrij axis
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.