Abstract
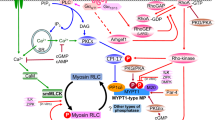
A rise in the concentration of cyclic AMP inhibits secretion in human platelets1–3. In mammalian cells the mechanism of action of cyclic AMP involves activation of a protein kinase and results in protein phosphorylation4. It has previously been shown that myosin light chain kinase purified from smooth muscle is a substrate for the catalytic subunit of cyclic AMP-dependent protein kinase5 and that phosphorylation of smooth muscle myosin kinase results in a decrease in its activity6. Here we present evidence that platelet myosin kinase is a substrate for the catalytic subunit of protein kinase and that phosphorylation of this myosin kinase, isolated from a non-muscle cell, decreases the activity of this enzyme. Dephosphorylation of platelet myosin kinase by a purified phosphatase7 restores its original activity. A decrease in myosin kinase activity in platelets would increase the relative amount of unphosphorylated myosin, which unlike phosphorylated myosin, cannot interact with actin8,9. These findings suggest a mechanism by which cyclic AMP might modulate actin–myosin interaction in platelets and other non-muscle cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Haslam, R. J. & Lynham, J. A. Biochem. biophys. Res. Commun. 77, 714–722 (1977).
Haslam, R. J. & Lynham, J. A. Thromb. Res. 12, 619–628 (1978).
Haslam, R. J., Lynham, J. A. & Fox, J. E. B. Biochem. J. 178, 397–407 (1979).
Krebs, E. G. & Beavo, J. A. A. Rev. Biochem. 48, 923–959 (1979).
Adelstein, R. S., Conti, M. A., Hathaway, D. R. & Klee, C. B. J. biol. Chem. 253, 8347–8350 (1978).
Conti, M. A. & Adelstein, R. S. J. biol. Chem. 256, 3178–3182 (1981).
Pato, M. D. & Adelstein, R. S. J. biol. Chem. 255, 6535–6538 (1980).
Adelstein, R. S. & Conti, M. A. Nature 256, 597–598 (1975).
Lebowitz, E. A. & Cooke, R. J. biol. Chem. 253, 5443–5447 (1978).
Hathaway, D. R. & Adelstein, R. S. Proc. natn. Acad. Sci. U.S.A. 76, 1653–1657 (1979).
Dabrowska, R. & Hartshorne, D. J. Biochem. biophys. Res. Commun. 85, 1352–1359 (1978).
Scordilis, S. P. & Adelstein, R. S. J. biol. Chem. 253, 9041–9048 (1978).
Trotter, J. A. & Adelstein, R. S. J. biol. Chem. 254, 8781–8785 (1979).
Yerna, M.-J., Aksoy, M. O., Hartshorne, D. J. & Goldman, R. D. J. Cell Sci. 31, 411–429 (1978).
Scholey, J. M., Taylor, K. A. & Kendrick-Jones, J. Nature 287, 233–235 (1980).
Kerrick, W. G. L., Hoar, P. E., Cassidy, P. S. & Malencik, D. A. Regulation of Muscle Contraction: Excitation–Contraction Coupling (eds Grinnell, A. D. & Brazier, M. A. B.) 227–239 (Academic, New York, 1980).
Daniel, J. L., Molish, I. R., Holmsen, H. & Salganicoff, L. Cold Spring Harb. Conf. Cell Proliferation Vol. 8 (in the press).
Walsh, D. A. et al. J. biol. Chem. 246, 1977–1985 (1971).
Klee, C. B. Biochemistry 16, 1017–1024 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hathaway, D., Eaton, C. & Adelstein, R. Regulation of human platelet myosin light chain kinase by the catalytic subunit of cyclic AMP-dependent protein kinase. Nature 291, 252–254 (1981). https://doi.org/10.1038/291252a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/291252a0
This article is cited by
-
Antioxidant and antiplatlet aggregation properties of bark extracts of Garcinia pedunculata and Garcinia cowa
Journal of Food Science and Technology (2014)
-
The critical roles of cyclic AMP/cyclic AMP-dependent protein kinase in platelet physiology
Frontiers of Biology in China (2009)
-
Functional and biochemical evidence of a specific adenosine A2/Ra receptor on human platelets *
La Ricerca in Clinica e in Laboratorio (1988)
-
Forskolin inhibits potassium-evoked release of vasopressin from rat neurohypophyses
Naunyn-Schmiedeberg's Archives of Pharmacology (1985)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.