Abstract
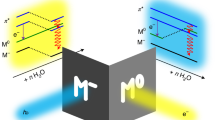
The observation that clusters of neutral H2O () or SO2 (ref. 5) molecules, on impact with essentially any solid surface, can decay efficiently into positively and negatively charged fragments has defied explanation, not least because the kinetic energy per molecule can be much smaller than the molecular ionization potentials. Here we present a microscopic model of the charging mechanism, based on a mass analysis of charged SO2 cluster fragments, which appears to be applicable to polar-molecule clusters more generally. Our mass spectra reveal that all positively charged fragments carry an alkali ion (sodium, potassium or caesium), whereas the negative fragments are simply (SO2)n−. The yields of both charged species are comparable, and can be enhanced significantly by pre-treating the sample surface with additional alkali atoms. The key to charge separation in the clusters therefore appears to be the pickup of a neutral (but readily ionized) adatom during impact, followed by delocalization of the adatom's valence electron within the cluster and the subsequent collision-induced fragmentation of the cluster into charged pieces. This process could be of practical use in, for example, charge-pair generation and surface analysis; it may also be relevant to atmospheric ionization processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Vostrikov, A. A., Dubov, D. Yu. & Predtechenskiy, M. R. Ionization of water clusters by surface collision. Chem. Phys. Lett. 139, 124–128 (1987).
Vostrikov, A. A. & Dubov, D. Yu. Surface induced ionization of neutral water clusters. Z. Phys. D 20, 61–63 (1991).
Vostrikov, A. A. et al. Ionization of water clusters by collision with surface. Z. Phys. D 40, 542–545 (1997).
Andersson, P. U. & Pettersson, J. B. C. Ionization of water clusters by collisions with graphite surfaces. Z. Phys. D 41, 57–62 (1997).
Christen, W., Kompa, K.-L., Schröder, H. & Stülpnagel, H. Ionization of SO2-clusters by scattering from surfaces. Ber. Bunsenges. Phys. Chem. 96, 1197–1200 (1992).
Hagena, O. F. & Obert, W. Cluster formation in expanding supersonic jets: effect of pressure, temperature, nozzle size and test gas. J. Chem. Phys. 56, 1793–1802 (1972).
Chandezon, F., Huber, B. & Ristori, C. Anew-regime Wiley-McLaren time-of-flight mass spectrometer. Rev. Sci. Instrum. 65, 3344–3353 (1994).
Gmelin Handbuch Schwefel, Ergänzungsband 3, 226 (Springer, Berlin, (1980).
Lee, G. H. et al. Negative ion photoelectron spectroscopy of solvated electron cluster anions, (H2O)−nand (NH3)−n. Z. Phys. D 20, 9–12 (1991).
Rothe, E. W., Tang, S. Y. & Reck, P. G. Measurement of electron affinities of O3, SO2, and SO3by collisional ionization. J. Chem. Phys. 62, 3829–3831 (1975).
Becker, C. H. & Gillen, K. T. Surface analysis of contaminated GaAs: Comparison of new laser-based techniques with SIMS. J. Vac. Sci. Technol. A 3, 1347–1349 (1985).
Tang, I. N., Lian, M. S. & Castleman, A. W. J Mass spectrometric study of gas-phase clustering reactions: Hydration of the monovalent strontium ion. J. Chem. Phys. 65, 4022–4027 (1976).
Donnelly, S. G. & Farrar, J. M. Size-dependent photodissociation cross sections for Sr+(NH3)n, n = 3–6: Rydberg state formation and electron transfer. J. Chem. Phys. 98, 5450–5459 (1993).
Weinheimer, C. J. & Lisy, J. M. Vibrational and unimolecular dissociation of mixed solvent cluster ions: Na+(CH3)2CO)n(CH3OH)m. Chem. Phys. 239, 357–368 (1998).
Ohshimo, K., Tsunoyama, H., Yamakita, Y., Misaizu, F. & Ohno, K. Photoionization and density functional study of clusters of alkali metal atoms solvated with acetonitrile molecules, M(CH3CN)n(M = Li and Na). Chem. Phys. Lett. 301, 356–364 (1999).
Takasu, R., Misaizu, F., Hashimoto, K. & Fuke, K. Microscopic solvation process of alkali atoms in finite clusters: photoelectron and photoionization studies of M(NH3)nand M(H2O)n(M = Li, Li−, Ma−). J. Phys. Chem. A 101, 3078–3087 (1997).
Hertel, I. V., Hüglin, C., Nitsch, C. & Schulz, C. P. Photoionization of Na(NH3)nand Na(H2O)nclusters: a step towards the liquid phase? Phys. Rev. Lett. 67, 1767–1770 (1991).
Barnett, R. N. & Landman, U. Hydration of sodium in water clusters. Phys. Rev. Lett. 70, 1775–1778 (1993).
Kim, K. S. et al. The nature of a wet electron. Phys. Rev. Lett. 76, 956–959 (1996).
Feller, D., Glendening, E. D., Kendall, R. A. & Peterson, K. A. An extended basis set ab initio study of Li+(H2O)n, n = 1–6. J. Chem. Phys. 100, 4981–4997 (1994).
Mosyak, A. A., Prezhdo, O. V. & Rossky, P. J. Solvation dynamics of an excess electron in methanol and water. J. Chem. Phys. 109, 6390–6395 (1998).
Rips, I. Electron solvation dynamics in polar liquids. Chem. Phys. Lett. 245, 79–84 (1995).
Jortner, J. Cluster size effects. Z. Phys. D 24, 247–275 (1992).
Christen, W., Even, U., Raz, T. & Levine, R. D. Collisional energy loss in cluster surface impact: Experimental, model, and simulation studies of some relevant factors. J. Chem. Phys. 108, 10262–10273 (1998).
Svanberg, M., Ming, L., Markovic, N. & Pettersson, J. B. C. Collision dynamics of large water clusters. J. Chem. Phys. 108, 5888–5897 (1998).
MacGorman, D. R. & Rust, W. D. The Electrical Nature of Storms 32 (Oxford Univ. Press, New York, (1998).
Pruppacher, H. R. & Klett, J. D. Microphysics of Clouds and Precipitation 813–814 (Kluwer Academic, Dordrecht, (1996).
Di Palma, T. M., Latini, A., Satta, M. & Giardini Guidoni, A. Molecular beam studies of ammonia clustered with metals produced by pulsed laser reactive ablation. Int. J. Mass Spectrom. 179/180, 319–326 (1998).
Acknowledgements
We thank R. D. Levine for discussions, and H.-J. Schmidtke for contributions to the early stage of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gebhardt, C., Schröder, H. & Kompa, KL. Surface impact ionization of polar-molecule clusters through pickup of alkali atoms. Nature 400, 544–547 (1999). https://doi.org/10.1038/22984
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22984
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.