Abstract
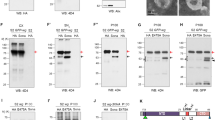
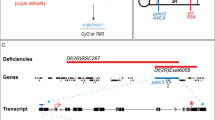
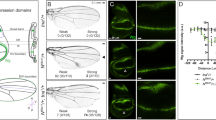
The Drosophila wingless gene (wg) encodes a protein of the Wnt family and is a critical regulator in many developmental processes1. Biochemical studies have indicated that heparan sulphate proteoglycans, consisting of a protein core to which heparan sulphate glycosaminoglycans are attached2, are important for Wg function3. Here we show that, consistent with these findings, the Drosophila gene sulfateless (sfl), which encodes a homologue of vertebrate heparan sulphate N -deacetylase/N -sulphotransferase (an enzyme needed for the modification of heparan sulphate) is essential for Wg signalling. We have identified the product of division abnormally delayed (dally), a glycosyl-phosphatidyl inositol (GPI)-linked glypican, as a heparan sulphate proteoglycan molecule involved in Wg signalling. Our results indicate that Dally may act as a co-receptor for Wg, and that Dally, together with Drosophila Frizzled 2, modulates both short- and long-range activities of Wg.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Siegfried, E. & Perrimon, N. Drosophila wingless: a paradigm for the function and mechanism of Wnt signaling. BioEssays 16, 395–404 (1994).
David, G. Integral membrane heparan sulfate proteoglycans. FASEB J. 7, 1023–1030 (1993).
Reichsman, F., Smith, L. & Cumberledge, S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J. Cell Biol. 135, 819–827 (1996).
Cumberledge, S. & Reichsman, F. Glycosaminoglycans and WNTs: just a spoonful of sugar helps the signal go down. Trends Genet. 13, 421–423 (1997).
Perrimon, N., Lanjuin, A., Arnold, C. & Noll, E. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144, 168–1692 (1996).
Haecker, U., Lin, X. & Perrimon, N. The Drosophila sugarless gene modulates. Wingless signaling and encodes an enzyme involved in polysaccharide biosynthesis. Development 124, 3565–3573 (1997).
Neumann, C. J. & Cohen, S. M. Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development 124, 871–880 (1997).
Zecca, M., Basler, K. & Struhl, G. Direct and long-range action of a wingless morphogen gradient. Cell 87, 833–844 (1996).
Hashimoto, Y., Orellana, A., Gil, G. & Hirschberg, C. B. Molecular cloning and expression of rat liver N-heparan sulfate sulfotransferase. J. Biol. Chem. 267, 15744–15750 (1992).
Nakato, H., Futch, T. A. & Selleck, S. B. The division abnormally delayed (dally) gene: a putative integral membrane proteoglycan required for cell division patterning during postembryonic development of the nervous system in Drosophila. Development 121, 3687–3702 (1995).
Bhanot, P. et al. Anew member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382, 225–230 (1996).
Kennerdell, J. R. & Carthew, R. W. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell 95, 1017–1026 (1998).
Zhang, J. & Carthew, R. W. Interactions between Wingless and DFz2 during Drosophila wing development. Development 125, 3075–3085 (1998).
Cadigan, K. M., Fish, M. P., Rulifson, E. J. & Nusse, R. Wingless repression of Drosophila wing development. Development 125, 3075–3085 (1998).
Schlessinger, J., Lax, I. & Lemmon, M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell 83, 357–360 (1995).
Jackson, S. M. et al. dally, a Drosophila glypican, controls cellular responses to the TGF-β-related morphogen, Dpp. Development 124, 4113–4120 (1997).
Bullock, S. L., Fletcher, J. M., Beddington, R. S. & Wilson, V. A. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 12, 1894–1906 (1998).
Sen, J., Goltz, J. S., Stevens, L. & Stein, D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal–ventral polarity. Cell 95, 471–481 (1998).
van de Wetering, M. et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88, 789–799 (1997).
Gustafson, K. & Boulianne, G. L. Distinct expression patterns detected within individual tissues by the GAL4 enhancer trap technique. Genome 39, 174–182 (1996).
Acknowledgements
We thank R. Carthew, I. Duncan, H. Nakato, R. Nusse, M. Peifer and S. Selleck for reagents; S. Selleck and S. Kerridge for exchange of information before publication; and E. Bach, D. Bilder, U. Haecker, K. Hong, A. Michelson, B. Mathey-Prevot and I. The for comments on the manuscript. This work is supported by the US Army Medical Research and Material Command (X.L.) and HHMI (N.P.).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lin, X., Perrimon, N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400, 281–284 (1999). https://doi.org/10.1038/22343
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/22343
This article is cited by
-
Signalling architectures can prevent cancer evolution
Scientific Reports (2020)
-
Gene expression analysis of potential morphogen signalling modifying factors in Panarthropoda
EvoDevo (2018)
-
Supramolecular assembly of KAT2A with succinyl-CoA for histone succinylation
Cell Discovery (2018)
-
Sol narae (Sona) is a Drosophila ADAMTS involved in Wg signaling
Scientific Reports (2016)
-
Cell-autonomous heparanase modulates self-renewal and migration in bone marrow-derived mesenchymal stem cells
Journal of Biomedical Science (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.