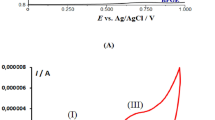
Abstract
ATTEMPTS to establish the reduction–oxidation potential of blood as a useful index in clinical medicine1 have met with little success. Possibly the redox measurement of blood, despite its reasonable reliability3, is too crude an index for the changes which are likely to occur in the many and complex interacting electron transport systems of the blood. On the other hand, the reasons for failure may be largely technical. Redox measurements of the blood have usually been carried out under unknown or uncontrolled conditions of pH. and temperature, and in the presence of unknown partial pressures of oxygen in the blood. These variables could account for relatively marked changes in the redox measurement2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ziegler, E., The Redox Potential of the Blood in Vivo and in Vitro (Charles C. Thomas, Springfield, Illinois, 1965).
Clark, W. M., Oxidation-Reduction Potentials of Organic Systems (Williams and Wilkins, Baltimore, Maryland, 1960).
Marmasse, C., and Grosz, H. J., Nature, 202, 95 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Grosz, H., FARMER, B. Reduction–Oxidation Potential of Blood as a Function of Partial Pressure of Oxygen. Nature 213, 717–718 (1967). https://doi.org/10.1038/213717a0
Issue Date:
DOI: https://doi.org/10.1038/213717a0
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.