Abstract
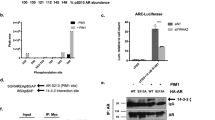
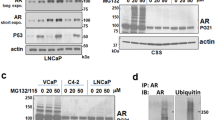
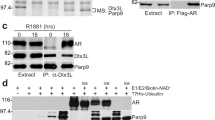
Androgen receptor signaling in prostate cancer cells is augmented by the androgen receptor (AR) coactivator p300, which transactivates and acetylates the AR in the presence of dihydrotestosterone (DHT). As prostate cancer (PC) cells progress to androgen independence, AR signaling remains intact, indicating that other factors stimulate AR activities in the absence of androgen. We previously reported that neuropeptide growth factors could transactivate the AR in the presence of very low concentrations of DHT. Here, we examine the involvement of p300 in neuropeptide activation of AR signaling. Transfection of increasing concentrations of p300 in the presence of bombesin into PC-3 cells resulted in a linear increase in AR transactivation, suggesting that p300 acts as a coactivator in neuropeptide-mediated AR transactivation. P300 is endowed with histone acetyltransferase (HAT) activity. Therefore, we examine the effect of bombesin on p300 HAT activity. At 4 h after the addition of bombesin, p300 HAT activity increased 2.0-fold (P<0.01). Incubation with neutral endopeptidase, which degrades bombesin, or bombesin receptor antagonists blocked bombesin-induced p300 HAT activity. To explore the potential signaling pathways involved in bombesin-induced p300 HAT activity, we examined Src and PKCδ pathways that mediate bombesin signaling. Inhibitors of Src kinase activity or Src kinase siRNA blocked bombesin-induced p300 HAT activity, whereas PKCδ inhibitors or PKCδ siRNA significantly increased bombesin-induced p300 HAT activity suggesting that Src kinase and PKCδ kinase are involved in the regulation of p300 HAT activity. As AR is acetylated in the presence of 100 nM DHT, we next examined whether bombesin-induced p300 HAT activity would result in enhanced AR acetylation. Bombesin-induced AR acetylation at the same motif KLKK observed in DHT-induced acetylation. Elimination of p300 using p300 siRNA reduced AR acetylation, demonstrating that AR acetylation was mediated by p300. AR acetylation results in AR transactivation and the expression of the AR-regulated gene prostate-specific antigen (PSA). Therefore, we examined bombesin-induced AR transactivation and PSA expression in the presence and absence of p300 siRNA and found inhibition of p300 expression reduced bombesin-induced AR transactivation and PSA expression. Together these results demonstrate that bombesin, via Src and PKCδ signaling pathways, activates p300 HAT activity which leads to enhanced acetylation of AR resulting in increased expression of AR-regulated genes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Aprikian AG, Han K, Guy L, Landry F, Begin LR, Chevalier S . (1998). Prostate 8: 52–61.
Aprikian AG, Tremblay L, Han K, Chevalier S . (1997). Int J Cancer 72: 498–504.
Bologna M, Festuccia C, Muz P, Biordi L, Ciomei M . (1989). Cancer 63: 1714–1720.
Borre M, Nerstrom B, Overgaard J . (2000). Clin Cancer Res 6: 1882–1890.
Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND et al. (2005). J Biol Chem 280 (11): 10264–10276.
Bubendorf L, Kolmer M, Kononen J, Koivisto P, Mousses S, Chen Y et al. (1999). J Natl Cancer Inst 91: 1758–1764.
Chan HM, La Thangue NB . (2001). J Cell Sci 114: 2363–2373.
Chim CS, Wong ASY, Kwong YL . (2004). Cancer Genet Cytogenet 150: 164–167.
Cui Y, Zhang M, Pestell RG, Curran EM, Welshons WV, Fuqua SAW . (2004). Cancer Res 64: 9199–9208.
Culig Z, Hobisch A, Bartsch G, Klocker H . (2000). Microsc Res Tech 51: 447–455.
Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trampman J, Hittmair A et al. (1994). Cancer Res 54: 5474–5478.
Culig Z, Klocker H, Bartsch G, Hobisch A . (2002). Endocr Relat Cancer 9: 155–170.
Dai J, Shen R, Sumitomo M, Stahl R, Navarro D, Gershengorn MC et al. (2002). Clin Cancer Res 8: 2399–2405.
Darne C, Vessiere G, Jean C . (1998). European J Biochem 256: 541–549.
Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ . (2005). Cancer Res 65: 5965–5973.
Debes JD, Schmidt LJ, Huang H, Tindall DJ . (2002). Cancer Res 62: 5632–5636.
Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DAL, Tindall DJ . (2003). Cancer Res 63: 7638–7640.
Fu M, Rao M, Wang C, Sakamaki T, Wang J, Di Vizio D et al. (2003). Mol Cell Biol 23: 8563–8575.
Fu M, Rao M, Wu K, Wang C, Zhang X, Hessien M et al. (2004a). J Biol Chem 279 (9): 29436–29449.
Fu M, Wang C, Reutens AT, Wang J, Angeletti RH, Siconolfi-Baez L et al. (2000). J Biol Chem 275: 20853–20860.
Fu M, Wang C, Wang J, Zafonte B, Lisanti MP, Pestell RG . (2002a). Cytokine Growth Factor Rev 13: 259–276.
Fu M, Wang C, Wang J, Zhang X, Sakamaki T, Yeung YG et al. (2002b). Mol Cell Biol 22: 3373–3388.
Fu M, Wang C, Zhang X, Pestell RG . (2004b). Biochem Pharmacol 68: 1199–1208.
Fu M, Wang CG, Rao M, Wu X, Bouras T, Zhang X et al. (2005). J Biol Chem 280: 29728–29742.
Gaughan L, Logan IR, Cook S, Neal DE, Robson CN . (2002). J Biol Chem 277: 25904–25913.
Gayther SA, Batley SJ, Linger L, Bannister A, Thorpe K, Chin SF et al. (2000). Nat Genet 24: 300–303.
Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD et al. (2002). J Biol Chem 277: 29304–29314.
Giordano A, Avantaggiati ML . (1999). J Cell Physiol 181: 218–230.
Heinlein CA, Chang C . (2002). Endocr Rev 23: 175–200.
Kang Z, Janne OA, Palvomo JJ . (2004). Mol Endocrinol 18: 2633–2648.
Kasper S, Rennie PS, Bruchovsky N, Lin L, Cheng H, Snoek R et al. (1999). J Mol Endocrinol 22: 313–325.
Kitabayashi I, Eckner R, Arany Z, Chiu R, Gachelin G, Liverston DM et al. (1995). EMBO J 14: 33496–33509.
Lee LF, Guan J, Qiu Y, Kung HJ . (2001). Mol Cell Biol 21: 8385–8397.
Levine L, Lucci III JA, Pazdrak B, Cheng JZ, Gu YS, Townsend Jr CM et al. (2003). Cancer Res 63: 3495–3502.
McKenna NJ, Lanz RB, O'Malley BW . (1999). Endocr Rev 20: 321–344.
Papandreou CN, Usmani B, Geng YP, Bogenrieder T, Freeman RH, Wilk S et al. (1998). Nat Med 4: 50–57.
Sadar MD, Hussain M, Bruchovsky N . (1999). Endocr Relat Cancer 6: 487–502.
Schwartz C, Beck K, Mink S, Schmolke M, Budde B, Wenning D et al. (2003). EMBO J 22: 882–892.
Shen R, Sumitomo M, Dai J, Hardy DO, Navarro D, Usmani B et al. (2000). Mol Cell Endocrinol 170: 131–142.
Shigeno K, Yoshida H, Pan L, Luo JM, Fujisawa S, Naito K et al. (2004). Cancer Lett 213: 11–20.
Sumitomo M, Milowsky MI, Shen R, Navarro D, Dai J, Asano T et al. (2001). Cancer Res 61: 3294–3298.
Sumitomo M, Shen R, Goldberg JS, Dai J, Navarro D, Nanus DM. . (2000a). Cancer Res 60: 6590–6596.
Sumitomo M, Shen R, Walburg M, Dai J, Geng Y, Navarro D et al. (2000b). J Clin Inv 106: 1399–1407.
Vashchenko N, Abrahamsson PA . (2005). Eur Urol 47: 147–155.
Vo N, Goodman RH . (2001). J Biol Chem 276: 13505–13508.
Wang C, Fu M, Pestell RG . (2004). Methods Mol Biol 241: 207–216.
Yaciuk P, Moran E . (1991). Mol Cell Biol 11: 5389–5397.
Yuan LW, Gambee JE . (2000). J Biol Chem 275: 40946–40951.
Yuan LW, Soh J, Weinstein IB . (2002). Biochem Biophys Acta 1592: 205–211.
Acknowledgements
This work was supported by NIH Grants RO1 DK060908-02, RO1 CA80240, Ronald and Susan Lynch Professorship in Urologic Oncology (to JG), Brady Urology Foundation of the Department of Urology and the Robert H McCooey Memorial Cancer Research Fund. The work was supported by NIH Grants: RO1CA86072, RO1CA93596-01 and RO1CA107382 (to RGP). Work conducted at the Lombardi Comprehensive Cancer Center was supported by National Institutes of Health Cancer Center Core Grant P30CA51008-14. We thank Dr Fu M for his kind assistance and helpful discussion on AR mutant studies. We acknowledge Drs Rong Zheng, Sandra Houser, Akio Horiguchi and Daniel Navarro for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gong, J., Zhu, J., Goodman, O. et al. Activation of p300 histone acetyltransferase activity and acetylation of the androgen receptor by bombesin in prostate cancer cells. Oncogene 25, 2011–2021 (2006). https://doi.org/10.1038/sj.onc.1209231
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1209231
Keywords
This article is cited by
-
Androgen receptor co-activators in the regulation of cellular events in prostate cancer
World Journal of Urology (2012)
-
Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases
Oncogene (2010)
-
Histone acetyltransferase inhibitory activity of Bokbunja (Rubus coreanus Miq.) ethanol extract on androgen receptor-dependent prostate cancer cell growth
Food Science and Biotechnology (2010)