Abstract
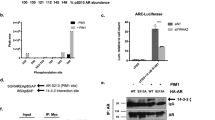
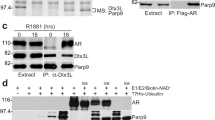
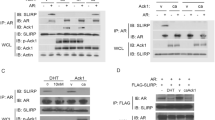
PIASy, a member of the protein inhibitor of activated STAT (PIAS) family, represses the transcriptional activity of the androgen receptor (AR). In this report, we investigate the mechanism of PIASy-mediated repression of AR. We show that AR binds to the RING-finger like domain of PIASy. PIASy contains two transcriptional repression domains, RD1 and RD2. RD1, but not RD2, is required for PIASy-mediated repression of AR. We show that the RD1 domain binds HDAC1 and HDAC2 and that HDAC activity is required for PIASy-mediated AR repression. PIAS proteins possess small ubiquitin-related modifier (SUMO) E3 ligase activity. Conjugation of SUMO-1 to AR has been implicated in the regulation of AR activity. We examine if the SUMO ligase activity of PIASy is required for PIASy to repress AR. We show that a mutant PIASy, defective in promoting sumoylation, retains the ability to repress AR transcription. In addition, mutation of all the known sumoylation acceptor sites of AR does not affect the transrepression activity of PIASy on AR. Our results suggest that PIASy may repress AR by recruiting histone deacetylases, independent of its SUMO ligase activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Aravind L and Koonin EV . (2000). Trends Biochem. Sci., 25, 112–114.
Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P and Shuai K . (1997). Science, 278, 1803–1805.
Emami KH and Carey M . (1992). EMBO J., 11, 5005–5012.
Gross M, Liu B, Tan J, French FS, Carey M and Shuai K . (2001). Oncogene, 20, 3880–3887.
Huang W, Shostak Y, Tarr P, Sawyers C and Carey M . (1999). J. Biol. Chem., 274, 25756–25768.
Iniguez-Lluhi JA and Pearce D . (2000). Mol. Cell Biol., 20, 6040–6050.
Johnson ES and Blobel G . (1999). J. Cell Biol., 147, 981–994.
Johnson ES and Gupta AA . (2001). Cell, 106, 735–744.
Kahyo T, Nishida T and Yasuda H . (2001). Mol. Cell, 8, 713–718.
Kotaja N, Aittomaki S, Silvennoinen O, Palvimo JJ and Janne OA . (2000). Mol. Endocrinol., 14, 1986–2000.
Kotaja N, Karvonen U, Janne OA and Palvimo JJ . (2002). Mol. Cell. Biol., 22, 5222–5234.
Laherty CD, Yang WM, Sun JM, Davie JR, Seto E and Eisenman RN . (1997). Cell, 89, 349–356.
Liu B, Liao J, Rao X, Kushner SA, Chung CD, Chang DD and Shuai K . (1998). Proc. Natl. Acad. Sci. USA, 95, 10626–10631.
Liu B, Gross M, ten Hoeve J, Shuai K . (2001). Proc. Natl. Acad. Sci. USA, 98, 3203–3207.
Melchior F . (2000). Annu. Rev. Cell Dev. Biol., 16, 591–626.
Mizushima S and Nagata S . (1990). Nucleic Acids Res., 18, 5322.
Moilanen AM, Karvonen U, Poukka H, Yan W, Toppari J, Janne OA and Palvimo JJ . (1999). J. Biol. Chem., 274, 3700–3704.
Nelson V, Davis GE and Maxwell SA . (2001). Apoptosis, 6, 221–234.
Nishida T and Yasuda H . (2002). J. Biol. Chem., 277, 41311–41317.
Ordentlich P, Downes M, Xie W, Genin A, Spinner NB and Evans RM . (1999). Proc. Natl. Acad. Sci. USA, 96, 2639–2644.
Poukka H, Karvonen U, Janne OA and Palvimo JJ . (2000). Proc. Natl. Acad. Sci. USA, 97, 14145–14150.
Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F and Grosschedl R . (2001). Genes Dev., 15, 3088–3103.
Schmidt D and Muller S . (2002). Proc. Natl. Acad. Sci. USA, 99, 2872–2877.
Shuai K and Liu B . (2003). Nature Rev Immunol, 3, 900–911.
Sternsdorf T, Jensen K, Reich B and Will H . (1999). J. Biol. Chem., 274, 12555–12566.
Tan J, Hall SH, Hamil KG, Grossman G, Petrusz P, Liao J, Shuai K and French FS . (2000). Mol. Endocrinol., 14, 14–26.
Tan JA, Hall SH, Hamil KG, Grossman G, Petrusz P and French FS . (2002). J. Biol. Chem., 277, 16993–17001.
Wu L, Matherly J, Smallwood A, Adams JY, Billick E, Belldegrun A and Carey M . (2001). Gene Ther., 8, 1416–1426.
Xu L, Glass CK and Rosenfeld MG . (1999). Curr. Opin. Genet. Dev., 9, 140–147.
Zamir I, Dawson J, Lavinsky RM, Glass CK, Rosenfeld MG and Lazar MA . (1997). Proc. Natl. Acad. Sci. USA, 94, 14400–14405.
Zhang L, Adams JY, Billick E, Ilagan R, Iyer M, Le K, Smallwood A, Gambhir SS, Carey M and Wu L . (2002). Mol. Ther., 5, 223–232.
Acknowledgements
We thank Bin Liu and Johanna ten Hoeve for comments and Michael Carey for providing plasmids and helpful discussions. Supported by grants from NIH (AI39612, CA32737), US Army Medical Research and Materiel Command (DAMD17-01-1-0036), and CaP CURE Foundation to KS and NIH (DK64379) to MG.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gross, M., Yang, R., Top, I. et al. PIASy-mediated repression of the androgen receptor is independent of sumoylation. Oncogene 23, 3059–3066 (2004). https://doi.org/10.1038/sj.onc.1207443
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1207443
Keywords
This article is cited by
-
Identification of pancreatic cancer type related factors by Weighted Gene Co-Expression Network Analysis
Medical Oncology (2020)
-
SUMO3 modification by PIAS1 modulates androgen receptor cellular distribution and stability
Cell Communication and Signaling (2019)
-
Protein Inhibitor of Activated STAT Y (PIASy) Regulates Insulin Secretion by Interacting with LIM Homeodomain Transcription Factor Isl1
Scientific Reports (2016)
-
PIAS4 is an activator of hypoxia signalling via VHL suppression during growth of pancreatic cancer cells
British Journal of Cancer (2013)
-
Yin Yang 1 regulates the transcriptional activity of androgen receptor
Oncogene (2009)