Abstract
Börjeson–Forssman–Lehman syndrome was first described in 1962. Many similar families and isolated cases have been reported since. In nineteen of them, including the original family, the clinical diagnosis was confirmed by the identification of a mutation in the responsible gene, PHF6. Summarizing recent clinical and molecular studies of this X-chromosome linked mental retardation syndrome we aim to offer a useful resource for its identification among the affected male and female subjects.
Similar content being viewed by others
In brief
-
BFLS is an uncommon, syndromic form of X-linked mental retardation.
-
Males are predominantly affected but milder manifestations may be seen in females.
-
Pregnancy and birth weight are normal but feeding problems and hypotonia are common in infancy.
-
Developmental delay is evident early; later intellectual handicap is mild to moderate.
-
Major physical features are large, fleshy earlobes, foreshortened toes and small genitalia.
-
Gynaecomastia, truncal obesity, tapered fingers and some coarsening of facial features emerge through childhood and adolescence.
-
Mutations in the zinc finger gene PHF6 are the cause of BFLS.
-
There is no evidence for genetic heterogeneity of BFLS.
-
Function of the PHF6 protein and molecular pathogenesis of BFLS is not known.
-
Management is symptomatic. Genetic counseling is indicated.
Clinical overview
Börjeson–Forssman–Lehman syndrome (BFLS) is a relatively uncommon type of syndromic X-linked mental retardation that has now been well described in 19 families and isolated cases with mutations in the responsible gene, PHF6.1, 2, 3 It is clear that the main clinical features evolve with age and show considerable variation both within and between families.4, 5
Pregnancy, delivery and birth weight are normal. Small genitalia and large ears may be evident at birth and many infants have generalized hypotonia and poor feeding. Developmental delay is usually evident before the first birthday; the eventual degree of mental handicap is mild to moderate. The head circumference is usually normal but macrocephaly and microcephaly occur. The ears are large with fleshy lobes. Moderate short stature is the rule but may be marked and, by contrast, some reach normal height. Truncal obesity emerges in late childhood and gynaecomastia in adolescence (see Figure 1). The genitalia remain small. The fingers are tapered and malleable. The feet are broad with foreshortened, often flexed, toes (see Figure 1). In adult life there is some coarsening of the facial features with prominence of the supraorbital ridges and deep-set eyes (Figure 1, panels a and b). Less common findings have been a mild generalized polyneuropathy,5 epilepsy, Perthes disease, hearing impairment, cleft lip and palate and hypopituitarism. Female heterozygotes may have learning problems and show some physical manifestations particularly shortened toes, thickened, fleshy ear lobes, pronounced supraorbital ridges and deep-set eyes. The pattern of inheritance is X linked.
Photographs of affected males with BFLS. Panels (a–d) show a BFLS patient with deep-set eyes, ptosis, prominent supraorbital ridges, large ears and earlobes, fleshy tapered fingers, a short fourth metatarsal with short flexed toes and a broad toe (from family F18, see Table 1). Panels (e–g) show three adolescent patients with gynaecomastia at the age of 12 years (from family F6, see Table 1) and two brothers 14- and 13-year-old (from family F16, see Table 1). Panels (e–g) were reproduced with kind permission from Blackwell Publishing Ltd from Clin Genet 2004 March; 65(3): 226–232.
Differential diagnosis
Many mentally retarded boys are obese but few have BFLS. The most helpful clinical diagnostic features are the long, fleshy earlobes; shortened, abnormal toes; tapered, malleable fingers, gynaecomastia and moderate obesity in adolescence and underdeveloped, external genitalia. This cluster of signs occurs in 90% or more of those boys where mutations in PHF6 are found. Other useful diagnostic features are moderate hypotonia and feeding difficulties in infancy, and, in adults, some coarsening of the facial appearance with deep-set eyes and prominent supraorbital ridges. In the early descriptions of this syndrome microcephaly and short stature were emphasized but although these do occur they are not common.
With a clear X linked pedigree the main differential diagnosis is the Coffin–Lowry syndrome (CLS; MIM #303600). However, in CLS, the characteristic facies appears early in life with hypertelorism, downslanting eyes, short nose and thick everted lips; the ears are not enlarged; height is less than the third centile; kyphoscoliosis and pectus excavatum and carinatum are common; mental retardation is more severe and occurs more often in heterozygotes; mutations in RSK2 can be demonstrated. Other, less well-defined, single families with X linked mental retardation and obesity have been described; in only one of these has the gene been mapped (Wilson–Turner syndrome; Xp21.1–q22; MIM #309585).
In the isolated male with BFLS, Klinefelter syndrome may be suggested but this syndrome lacks the tapered fingers and short toes and has an abnormal karyotype. The Prader–Willi syndrome (MIM #176270) has some resemblances but neonatal hypotonia and feeding problems are more severe, subsequent hyperphagia is distinctive, obesity is more extreme and the characteristic fingers and toes are lacking. Isolated heterozygote females might be mistaken for CLS or pseudohypoparathyroidism.
Gene
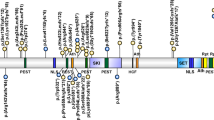
The PHF6 gene was identified in 2002 by Lower et al.1 It is a member of a large family of zinc-finger genes. PHF6 is transcribed as a ∼4.5 kb mRNA, which shows a ubiquitous expression pattern as tested by Northern blot hybridization1 and RT PCR.6 Two major isoforms exist. These differ in the inclusion or exclusion of intron 10 from the mRNA. Although this alternative splicing does not change the PHF6 protein it might be involved in the regulation of PHF6 mRNA stability and/or translation. However, this is only speculation, which needs further experimental substantiation.1 More recently, Landais et al6 reported the existence of six other isoforms of the Phf6 gene in mouse. Their function is not clear although five of them (t1, t4, t5, t6 and t7) can potentially give rise to alternative Phf6 protein isoforms. These new Phf6 mRNA isoforms are infrequent (<2%) and identified only by analysis of ESTs without further validation.
The PHF6 gene is highly conserved in vertebrates, but has no obvious ortholog(s) in lower organisms (ie, insects or yeast).1 Its ubiquitous expression pattern together with high conservation, and in particular, within the plant homeodomain (PHD) finger domains, suggests an important cellular role.
PHF6 mutations and mutation screening
There are 19 unrelated cases of BFL syndrome with confirmed PHF6 gene mutations reported in the literature.1, 2, 3, 5, 7, 8 Among these are 13 patients with a positive family history, including the original family described by M Börjeson, H Forssman and O Lehmann in 1962,10 and six isolated cases. These mutations are summarized in Table 1. Overall there are 12 different mutations found, predominantly missense and truncation mutations. Five of these mutations are recurrent, with the c.1024C>T/p.R342X truncating mutation being the most frequent, found in 4 out of 19 cases (21%). The PHF6 mutations do not seem to cluster although mutations in or around exon 2 and in exon 10 account for 13/19 (68.4%) mutations found so far. Where it was possible to investigate this,1, 5 it was confirmed that identical mutations are not identical by descent and arose independently. Some of these mutations, like c.27_28insA/G10fsX21 (case 3 of Crawford et al2) or c.1024C>T/p.R342X (family 3 of Lower et al5) arose de novo (likely on the paternal chromosome as experimentally confirmed for case 3 of Crawford et al2).
Current2 and past1, 5 experience with mutation screening tells us that there are no known familial cases with clinically diagnosed BFL syndrome where mutations in the PHF6 gene were not found. This, together with the identification of the PHF6 mutation in the original BFLS family5, 10 suggests that unlike many other XLMR syndromes11 BFLS is not genetically heterogeneous. However, this does not mean that clinical and molecular diagnoses are well aligned as PHF6 mutation pickup rate in clinically diagnosed individuals with BFLS (predominantly isolated cases, 20/25) is relatively low, 5/25 cases (20%).2
When considering screening for PHF6 gene mutations in patients with suspected clinical diagnosis of BFLS it might be worthwhile to consider the family history (as for any other X-linked syndrome) and X-inactivation skewing in the patient (if female) or the mother of the patient (if male). One has to acknowledge that de novo mutations will go undetected if these criteria were applied strictly, but a positive family history and X-inactivation skewing would provide an additional incentive to screen for PHF6 in patients where the clinical picture is not clear cut. Presently there is no evidence to suggest that mutations in PHF6 cause other syndromic or nonsyndromic X-linked mental retardation or phenotypes other than BFLS.
X-inactivation skewing in carrier females
The status of X inactivation in obligate carrier females of PHF6 mutations has recently been discussed.2 It appears that although it is much more likely for a PHF6 mutation carrier female to have skewed (>70%; three families) or highly skewed (>90%; seven families) X inactivation, there are also families (three families) where the X inactivation is random.2 There is no obvious correlation between a particular PHF6 mutation and X-inactivation skewing and X inactivation can vary among the members of the same family. Nor is there a clear cut correlation between the X-inactivation skewing (as measured almost invariably on white blood cells) and the variability of clinical presentation of the BFLS phenotype in carrier females. The only female patient diagnosed with BFLS and confirmed PHF6 mutation (case #3 of Crawford et al) had highly skewed X inactivation in peripheral blood cells with an estimated 93% of the cells expressing the normal PHF6 allele.2 Similar levels of X-inactivation skewing were identified in several other BFLS carriers although their phenotype was mostly normal or very mild.2, 4 These observations suggest that the level of X-inactivation skewing as measured in peripheral blood cells is currently not a reliable predictor of the penetrance of the BFLS features in obligate carriers of PHF6 mutations. Ascertainment of new cases will help to resolve this issue.
Function of the protein
PHF6 is a relatively small, unremarkable protein of 365 amino acids. It contains two similar PHD fingers.1 Similar PHD fingers are found in proteins involved in regulation of transcription function (eg MLL, MLL2, MLL4, PHF11; J. Gécz, unpublished data), which was the function also proposed for PHD fingers.11 Interestingly, there is only one PHF6 mutation known, which directly affects critical residue of the first PHD finger, cytosine at position 99 (C99F).1 In addition to the PHD fingers the PHF6 protein also contains multiple (at least four clearly recognizable and functional)1, 6 nuclear localization sequences. Subcellular localization studies show that the PHF6 protein is localized in the cell nucleus and more prominently in the nucleolus.1, 6, 8 Traditionally, this organelle has been known as the ribosome assembly factory. However, as our knowledge advances it has been suggested that the nucleolus plays an important role, among others, in nuclear export, sequestering regulatory molecules, modifying small RNAs, cell cycle regulation and processes of aging.12, 13 The role of the PHF6 protein in the nucleolus is not known. More recently, mouse Phf6 protein was found overexpressed in tumors with rearrangements (radiation leukemia virus, RadLV integration) in the neighboring Kis2 locus (noncoding RNA; Unigene cluster Mm.277876, ∼250 kb proximal to the Phf6 gene).6 Overall there is very little information on the function of the PHF6 protein available. If we dare to speculate, based on the X-inactivation skewing in carrier females,1, 2 localization of the PHF6 protein to the cell nucleus and nucleolus1, 6, 8 and the latest observations of the Phf6 protein overexpression in specific mouse tumors,6 we would suggest an important role for PHF6 in cell growth and proliferation, which may be achieved via its participation in ribosome biogenesis (at the RNA and/or protein level).
Management
There is no specific treatment. Special education is required from early life and adults require a variable degree of supervision. Sexual activity is minimal but strong social relationships can be formed. Symptomatic treatment may be needed for seizures, Perthes disease and hearing impairment. In individual patients, a case may be made for bilateral mastectomy and/or testosterone replacement therapy. The family requires genetic counseling for X linkage and the information that identification of female heterozygotes and prenatal diagnosis are now possible.
Electronic database information and accession numbers
MIM was accessed at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM; PHF6 gene & protein information was accessed at Entrez Gene http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene; BFLS, MIM no. 301900; PHF6 gene, MIM no. 300414, GenBank nos. NM_001015877, NM_032335 and NM_032458.
Accession codes
References
Lower KM, Turner G, Kerr BA et al: Mutations in PHF6 are associated with Börjeson–Forssman–Lehmann syndrome. Nat Genet 2002; 32: 661–665.
Crawford J, Lower KM, Hennekam RC et al: Mutation screening in Börjeson–Forssman–Lehmann Syndrome (BFLS): identification of a novel, de novo PHF6 mutation in a female patient. J Med Genet 2005; July 25: ; [E-pub ahead of print].
Just W, Mucke J : Towards a genotype-phenotype correlation in individuals with Börjeson–Forssman–Lehmann Syndrome. ESHG Meeting Abstract P0631 2005, Prague.
Turner G, Lower KM, White SM, et al: The clinical picture of the Börjeson–Forssman–Lehmann syndrome in males and heterozygous females with PHF6 mutations. Clin Genet 2004; 65: 226–232.
Lower KM, Solders G, Bondeson ML et al: 1024C>T (R342X) is a recurrent PHF6 mutation also in the original Börjeson–Forssman–Lehmann syndrome family. Eur J Hum Genet 2004; 12: 787–789.
Landais S, Quantin R, Rassart E : Radiation leukemia virus common integration at the Kis2 locus: simultaneous overexpression of a novel noncoding RNA and of the proximal Phf6 gene. J Virol 2005; 79: 11443–11456.
Baumstark A, Lower KM, Sinkus A et al: Novel PHF6 mutation p.D333del causes Börjeson–Forssman–Lehmann syndrome. J Med Genet 2003; 40: e50.
Vallee D, Chevrier E, Graham GE et al: A novel PHF6 mutation results in enhanced exon skipping and mild Börjeson–Forssman–Lehmann syndrome. J Med Genet 2004; 41: 778–783.
Börjeson M, Forssman H, Lehmann O : An X-linked, recessively inherited syndrome characterized by grave mental deficiency, epilepsy, and endocrine disorder. Acta Med Scand 1962; 171: 13–21.
Frints SG, Froyen G, Marynen P, Fryns JP : X-linked mental retardation: vanishing boundaries between non-specific (MRX) and syndromic (MRXS) forms. Clin Genet 2002; 62: 423–432.
Aasland R, Gibson TJ, Stewart AF : The PHD finger: implication for chromatin-mediated transcriptional regulation. Trends Biochemm Sci 1995; 20: 56–59.
Olson MOJ, Dundr M, Szebeni A : The nucleolus: old factory with unexpected capabilities. Cell Biol 2000; 10: 189–196.
Visitin R, Amon A : The nucleolus: the magician's hat for cell cycle tricks. Curr Opinion Cell Biol 2000; 12: 372–377.
Acknowledgements
This work was carried out with the support of the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gécz, J., Turner, G., Nelson, J. et al. The Börjeson–Forssman–Lehman syndrome (BFLS, MIM #301900). Eur J Hum Genet 14, 1233–1237 (2006). https://doi.org/10.1038/sj.ejhg.5201639
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201639
Keywords
This article is cited by
-
The Role of PHF6 in Hematopoiesis and Hematologic Malignancies
Stem Cell Reviews and Reports (2023)
-
Börjeson–Forssman–Lehmann syndrome: delineating the clinical and allelic spectrum in 14 new families
European Journal of Human Genetics (2023)
-
Loss of PHF6 leads to aberrant development of human neuron-like cells
Scientific Reports (2020)
-
The sub-nucleolar localization of PHF6 defines its role in rDNA transcription and early processing events
European Journal of Human Genetics (2016)
-
1H, 13C and 15N resonance assignments and secondary structure of the human PHF6-ePHD1 domain
Biomolecular NMR Assignments (2016)