Abstract
Faciogenital dysplasia or Aarskog–Scott syndrome (AAS) is a genetically heterogeneous developmental disorder. The X-linked form of AAS has been ascribed to mutations in the FGD1 gene. However, although AAS may be considered as a relatively frequent clinical diagnosis, mutations have been established in few patients. Genetic heterogeneity and the clinical overlap with a number of other syndromes might explain this discrepancy. In this study, we have conducted a single-strand conformation polymorphism (SSCP) analysis of the entire coding region of FGD1 in 46 AAS patients and identified eight novel mutations, including one insertion, four deletions and three missense mutations (19.56% detection rate). One mutation (528insC) was found in two independent families. The mutations are scattered all along the coding sequence. Phenotypically, all affected males present with the characteristic AAS phenotype. FGD1 mutations were not associated with severe mental retardation. However, neuropsychiatric disorders, mainly behavioural and learning problems in childhood, were observed in five out of 12 mutated individuals. The current study provides further evidence that mutations of FGD1 may cause AAS and expands the spectrum of disease-causing mutations. The importance of considering the neuropsychological phenotype of AAS patients is discussed.
Similar content being viewed by others
Introduction
Faciogenital dysplasia or Aarskog–Scott syndrome (AAS) is an inherited condition phenotypically characterised by distinct craniofacial dysmorphism (hypertelorism, down-slanting palpebral fissures), brachydactyly, urogenital abnormalities and disproportionate acromelic short stature. It was first described as a distinct disorder by Aarskog1 in 1970 and further delineated by Scott2 in 1971. The gene responsible for the X-linked form (MIM#305400), FGD1, was identified and characterised by positional cloning in a family in which the phenotype was associated with a balanced X-autosome translocation.3 The FGD1 gene encodes a guanine nucleotide exchange factor (GEF) for members of the Rho/Rac family of small GTP-binding proteins, binding specifically to the Rho GTPase Cdc42 and stimulating the GDP–GTP exchange of the isoprenylated form of Cdc42.4 Studies suggest that an FGD1/Cdc42 signalling pathway is involved in the cytoskeletal organisation and, ultimately, in the skeletal formation and morphogenesis.5
In the past, the clinical diagnosis of AAS has been made in a relatively large number of males based on the clinical dysmorphological findings. However, although recent studies have successfully demonstrated the feasibility of molecular diagnosis, to date only a limited number of mutations have been reported in AAS patients6,7,8 (Table 1). The genetic heterogeneity of the disorder and the overlapping clinical features of AAS with other unrelated conditions might explain this discrepancy. In addition to X-linked inheritance, there are various reports in which the family findings are compatible with autosomal-dominant9 (MIM#100050) or autosomal-recessive inheritance10 (MIM#227330). Furthermore, syndromes that are known to be genetically different, such as Noonan's syndrome, Short's syndrome, pseudohypoparathyroidism and Robinow's syndrome, present a considerable phenotypic overlap with AAS. Consequently, clinically based epidemiological studies that estimate a high prevalence of AAS need to be considered with caution as diagnostic confusion could account for ascertainment bias.11 In addition, it has been reported that some degree of cognitive impairment may be as high as 30% in the AAS subjects,12 while other studies reject the association with mental handicap.13 As a further confounding issue, a recent report of nonsyndromic XLMR associated to the P312L FGD1 mutation8 gives additional evidence that mental impairment may be part of the phenotype, even in the absence of the typical AAS features.
To increase diagnostic reliability, we performed a mutational analysis of the FGD1 gene in 46 familial and sporadic AAS males and compared these results with the individual phenotype. As in the previously reported studies,6,7 in only a minority of patients with the clinical diagnosis of AAS was an FGD1 mutation found. We detected eight FGD1 mutations, all novel, in nine out of 46 probands, accounting for 19.56% of the patients (Tables 1 and 2). Seven of these mutations were unique. Only one mutation was found to occur in two independent families.
Subjects, materials and methods
Subjects
The study included 46 independent AAS male probands, from genetics centres of different countries (Belgium, France, Germany, Ireland, Israel, Italy, Portugal, Spain, Switzerland). Their identification numbers were given according to the DNA database. The phenotype was evaluated through accurate clinical genetic examination and anthropometric measurements, and diagnosis of AAS was based on the primary and secondary diagnostic criteria as derived by Teebi et al.10 The majority of patients (42/46) are sporadic. Four families had more than one affected member. In the family with identical twins (patients 90 and 91), one number was considered as the proband. Genomic DNA from patients, family members where available, and 200 unrelated unaffected males used for control purposes was extracted from peripheral blood leucocytes by standard procedures. In the case of a positive result, molecular testing was offered to the other family members potentially at risk for carrying the same mutation.
Mutational analysis
Exons 1–18 of the FGD1 gene and the promoter region were amplified by PCR to give products in the size range 150–250 bp suitable for the subsequent single-strand conformational polymorphism (SSCP) analysis.7 Primer sequences were designed to span the intron/exon boundary regions of the published sequence of the gene (GeneBank Sequence: U11690). PCR reactions were performed in 25 μl containing 100 ng of genomic DNA, 1 × PCR buffer (Perkin-Elmer), 0.5 pmol of each primers, 180 μ M of each dNTP and 1 U of Taq DNA polymerase (Perkin-Elmer). Amplified samples were diluted 1:1 in formamide buffer (95% formamide, 10 mM NaOH, 0.025% bromophenol blue and 0.025% xylene cyanol), denatured at 95°C for 5 min and cooled on ice. A measure of 4–8 μl of PCR products were loaded onto nondenaturating polycrylamide gel containing 6% acrylamide prepared with a 99:1 ratio between acrylamide and bis-acrylamide. In order to better distinguish aberrant conformers, gels were run in two different conditions: at 4°C for 1–3 h at 45 W and at room temperature for 1–2 h at 40 W. DNA bands were visualised by silver staining. Fragments showing mobility shifts of single strands and/or heteroduplex formation were first reconfirmed by an independent PCR and then purified using Qiagen purification columns according to the manufacturing instructions and both strands were sequenced directly using the Sequenase 2.0 kit (USB).
Clinical data and molecular results
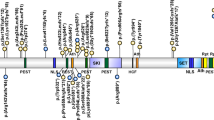
We identified eight novel mutations in nine of the 46 probands (19.56%), present neither in 200 normal control chromosomes nor in any of the unaffected family members analysed (Table 1). All the mutations were found to be family specific with the exception of an insertion in the exon 3 (528insC) that was detected in two independent families. The majority (5/8, including one insertion and four deletions) led to a premature termination codon and three were missense mutations.
Patient 53 was born by a full-term spontaneous vaginal delivery with uneventful perinatal history. Family history was negative. Birth weight was 3350 g. At the age of 1.3 years, the child presented an unusual facial appearance and short stature. Weight was 10.10 kg (25th percentile), height 76 cm (10th percentile) and head circumference was 45.5 cm (5th percentile). He presented with hypertelorism (inner canthal distance 3.9 cm; >97th percentile – outer canthal distance 11.5 cm; >97th percentile), antimongoloid slant of palpebral fissures, widow's peak, small nose with anteverted nares, long philtrum, cutaneous syndactyly with brachydactyly and clinodactyly of the Vth fingers, transverse palmar crease (Figure 1). The child also had a shawl scrotum, right cryptorchidism, a small right inguinal hernia and an umbilical hernia.
Molecular analysis revealed a single nucleotide deletion (982delC; exon 4). This mutation resulted in a frameshift after codon 327 and a predicted premature termination at the codon 359. The 982delC mutation was not present in his phenotypically normal mother. As he is the only affected member in his family, the mutation possibly arose de novo or from a maternal germline mosaicism.
Patient 61 was a 21-year-old man with clinically unaffected parents and sister. He was born at term, but pregnancy was complicated by oligohydramnios. His birth weight and length medical records were not available. The diagnosis of AAS was made on a clinical basis in childhood as he revealed a dysmorphic facial appearance, shawl scrotum and short stature. His adult height was 163 cm (3rd percentile). He presented hypertelorism (inner canthal distance 3.9 cm; >97th percentile – outer canthal distance 11.5 cm; >97th percentile), left palpebral ptosis and strabismus, a short neck, camptodactyly and brachydactyly. Attention and behavioural difficulties were reported during school age, while difficulties with organisational skills and social interaction became evident in adulthood. Molecular investigation revealed a 32 bp deletion mutation (944–975del32; exon 4) that resulted in a frameshift after codon 314 and a predicted premature termination at the codon 325. Also, this mutation most probably arose de novo as it was not present in his mother and sister.
Patient 26 was the proband of a family in which there was evidence of a family trait of short stature and brachydactyly consistent with X-linked inheritance. At the age of 16 months, his weight was 10.75 kg (25th percentile), height 78 cm (10th percentile), head circumference 46 cm (10th percentile), inner canthal distance 3 cm (90th percentile) and outer canthal distance was 8.5 cm (90th percentile). At the age of 3 years, he was diagnosed as having AAS based on the following features: round face, hypertelorism, epicanthic folds, widow's peak, small nose with anteverted nares, long philtrum, cutaneous syndactyly with brachydactyly and clinodactyly of the IVth and Vth fingers, right inguinal hernia, umbilical hernia, shawl scrotum, short stature and bilateral varus metatarsus. Molecular analysis revealed a single nucleotide change in exon 5 (1139A>C), predicted to generate an E380A substitution. Subsequent mutational analysis in other individuals from the family identified the same nucleotide alteration in his mother (patient 47) and maternal aunt (patient 48), as well as in his affected cousin (patient 27, son of patient 48) and maternal grandmother (patient 46). The affected cousin is an 18-month-old boy presenting with similar clinical features: round face, hypertelorism, down-slanting palpebral fissures, widow's peak, small nose with anteverted nares, long philtrum, brachydactyly with interdigital webbing, inguinal and umbilical hernia that required surgical repair, shawl scrotum and short stature. Both affected males had normal cognitive development. The three carrier females had a milder phenotype with widow's peak, short stature and brachydactyly.
Patient 73 was a 4.5-year-old affected male, born after normal pregnancy and delivery. His birth weight was 3.4 kg, length 49 cm and head circumference 33 cm. At birth he was noted to have short limbs and left-sided cleft lip. Psychomotor development was normal. At the age of 22 months, skeletal maturation was delayed (15 months). At the age of 3 years, his weight was 15 kg (50th percentile), height 94.5 cm (50th percentile), head circumference 48 cm (10th percentile), inner canthal distance 3.3 cm (90th percentile) and outer canthal distance 8.5 cm (>97th percentile). The dermatoglyphics on the right and left digits were: UL, A, A, A and UL. The clinical diagnosis of AAS was based on the presence of facial dysmorphic features (widow's peak, hypertelorism, epicanthic folds, down-slanting palpebral fissures with bilateral palpebral ptosis, short and broad nose with anteverted nostrils) associated with IVth and Vth fingers camptodactyly, shawl scrotum and inguinal hernias. At the time of our investigation, there was mild short stature (105 cm; 10th percentile), mild hyperextensible joints and posteriorly rotated low set ears. Mental development was normal and no behavioural problems were reported. Molecular analysis identified a missense mutation in exon 6 (1328 G>A) resulting in an R443H substitution. This single base change abolishes a CfoI restriction site present in the wild-type sequence allowing the rapid detection of the mutation. No other members of this family were available for further investigations.
Two affected siblings of healthy nonconsanguineous parents, patients 58 and 59, 10 and 7 years old, respectively, were examined as they both presented with a similar clinical history and phenotype. Patient 58, the proband, was born at term by C-section because of breech presentation with a birth weight of 3.3 kg and length of 46 cm. No perinatal problems were noted. The infant was in good condition at birth but he was hypotonic during the following months with mildly delayed developmental milestones (neck holding at 4 months, sitting with support at 8 months, walking at 19 months, first words at 12 months). At the time of the examination, he presented with short stature (125 cm; 3rd percentile) and mild learning and behavioural disabilities (hyperactivity and attention deficit). Clinical examination revealed hypertelorism (inner canthal distance 3.5 cm; 97th percentile – outer canthal distance 9.5 cm; 97th percentile), left palpebral ptosis, short nose with anteverted nostrils, long philtrum, posteriorly angulated low-set ears, prominent umbilicus, hyperextensible joints and brachydactyly with cutaneous II–V syndactyly. Patient 59, the youngest brother, was born at 42 weeks by C-section. His birth weight was 3.42 kg. He had, like his brother, mildly delayed developmental milestones (neck holding at 3 months, sitting without support at 6 months, walking at 18 months, first words at 18 months). His facial features were very similar to his brother's, with long face, telecanthus (inner canthal distance 3.7 cm; >97th percentile – outer canthal distance 8.5 cm; 80th percentile), short nose with anteverted nostrils, long philtrum, micrognatia and posteriorly angulated low-set ears. He had short stature (105 cm; <3th percentile), pectus excavatum, hyperextensible joints, brachydactyly with cutaneous I–V syndactyly, single palmar creases, hypospadias and right cryptorchidism. Although both brothers had neurodevelopmental delay in early childhood, at present they are referred to as mentally normal, with normal IQ scores (Raven Standard Progressive Matrices). Their mother presented with hypertelorism, brachydactyly and low-set ears. Molecular analysis revealed a 4 bp deletion mutation in exon 6 (1326–1319del AGCT) that resulted in a frameshift after codon 439 and a predicted premature translation termination at the codon 470 in both brothers. This mutation was inherited, as expected, from their mother who was found to be heterozygous.
Patient 65, a 16-year-old boy from Ireland, was born following a normal pregnancy. He had a full-term normal delivery and his birth weight was 3.4 kg. At birth, he was noted to have brachydactyly and camptodactyly, abnormal feet and shawl scrotum with bilateral undescended testicles. At the time of the investigation, he presented with hypertelorism (measurements are not available), slight downward slant palpebral fissures, short nose, broad philtrum, broad mouth, short broad hand, shawl scrotum with bilateral scars from previous orchidopexies. The patient has progressed through puberty satisfactorily attaining a normal height (175 cm). Intelligence was normal. Molecular analysis revealed a single nucleotide deletion in exon 17 (2530del G). This mutation resulted in a frameshift after codon 843 with a predicted premature termination at the codon 862. Other members of his family were not available for further investigations, although his maternal uncle and his mother's sister son are clinically affected.
Patient 50 and his family were previously reported.12 His facial features represent one of the most impressive and characteristic pictures of the AAS facial phenotype. He was a 29-year-old man of low normal intelligence (WISC-R scale: IQ 82). His weight was 75 kg and height was 160.5 cm. He was socially well integrated, married, and no behavioural problems were noted. At the age of 13 years a hydrocoele communicans was corrected. Oligoasthenozoospermia was found in the spermatogram. Molecular analysis revealed a single nucleotide insertion in exon 3 (528insC) that caused a frameshift after codon 176 and a predicted premature termination at codon 216. Further molecular analysis of family members showed a heterozygote pattern in his normal mother and sister. No mutation was found in the unaffected brother.
The same insertion (528insC) was also detected in a pair of identical twins of an apparently unrelated Italian family, patients 90 and 91. Both males, at the age of 9 years, had short stature (121 cm <5th percentile), joint hypermobility, small and short hands and feet, brachydactyly and camptodactyly (patient 90), interdigital webbing, inguinal hernia, shawl scrotum and a round face with a broad forehead and widow peak, hypertelorism (inner canthal distance 3.8 cm; >97th percentile – outer canthal distance 10 cm; >97th percentile), down-slanting palpebral fissures (patient 91) and dysmorphic ears. In this family, three generations were available for study. The 528insC was present in the mother, the maternal grandmother, one affected uncle and two maternal aunts. All female carriers expressed minor phenotypical manifestations such as short stature and interdigital webbing. Intelligence was normal in both the affected males and carrier females.
Patient 25 was a 11-year-old Portuguese boy. Pregnancy and delivery were normal. His birth weight was 3.6 kg, length 51 cm and head circumference 36 cm. He was referred to the Genetic Clinic at the age of 8 months because of clinical findings of slightly delayed developmental milestones, cardiac defect (ventricular septum defect), right cryptorchidism and facial dysmorphic features (Figure 2). At present, he is of low normal intelligence (WISC-R scale: IQ 80) with impairment in expressive language and speech articulation difficulties, emotional behaviour problems with occasional temper tantrums, short stature (132.5 cm; 5th percentile), moderate rhizomelia of upper and lower limbs, short hands with cutaneous syndactyly of fingers, a surgically corrected right-sided cryptorchidism and over-riding scrotum. Craniofacial findings included relative macrocephaly (OFC 55 cm; 90th percentile), frontal bossing, hypertelorism (inner canthal distance 3.5 cm; >97th percentile – outer canthal distance 11 cm; >97th percentile), down slant of palpebral fissures, short nose with anteverted nostrils, wide philtrum and thin upper lip. In the past, the chromosomal analysis in peripheral blood lymphocytes had revealed a 46,XY(90%)/47,XYY(10%) mosaic. Molecular analysis revealed a missense mutation in exon 3 (614 G>T) that causes a change in the residue 205 from Ser to Ile. The same mutation was found in his mother and in her three sisters, who have mild phenotypical signs (hypertelorism, widow's peak).
The patients in whom a mutation was not found (37 individuals) present with various combinations of short stature, facial appearance (hypertelorism, small nose with anteverted nares, broad nasal bridge, ptosis, strabismus), hand abnormalities and genitourinary manifestations (Table 2). Most clinical signs were concordant with the diagnosis. Short stature (34 out of 37 patients), hypertelorism (36 out of 37), hand abnormalities (short and broad hands, clinodactyly, camptodactyly) (28 out of 37) and the peculiar scrotal morphology (28 out of 37) were displayed by most of patients. Cryptorchidism was found in eight individuals and hernias in five. Interphalangeal webbing was recorded in 19 out of 37 patients. Only six had palpebral ptosis. A total of 12 patients presented severe mental retardation.
Discussion
At present, the diagnosis of AAS is primarily based on clinical criteria. In typical cases, the phenotype of affected males is characterised by genital anomalies (shawl scrotum, cryptorchidism), short stature, distinct craniofacial abnormalities, brachydactyly with interdigital webbing and joint laxity. A broad range of mild developmental delay or learning difficulty has occasionally been reported. Nevertheless, in affected males the phenotype is variable as they may exhibit different combinations of associated features. In general, carrier females may have a milder phenotype than males, showing minor and mild clinical signs, possibly depending on the pattern of X-chromosome inactivation. Despite the presence of clinical inclusion criteria and the advances in the molecular pathogenesis of AAS, disease-causing mutations have been identified in only a small number of patients. Possibly, both the variability of phenotype and the genetic heterogeneity account for a clinical overdiagnosis. Short stature with hypertelorism and brachydactyly represent a relatively frequent association in clinical dysmorphology. Moreover, AAS patients are often referred with various degrees of mental handicap (mild mental retardation, learning disabilities, attention-deficit disorders) and, as the majority of cases are sporadic, X-linked inheritance may be questionable.
In the present study, we performed mutation screening of the FGD1 gene in 46 male patients referred with the clinical diagnosis of AAS. This is the largest series reported to date. We identified eight mutations, all novel, including four deletions, one insertion and three missense mutations. The majority of the mutations identified were found to be unique to a single family. The only exception is the 528insC, occurring in exon 3, which was detected in two independent families (Belgian and Italian). The deletions and the insertion mutations are all predicted to result in a frameshift, which leads to a truncation of the protein. The three missense mutations, S205I, E380A and R443H, occur in exons 3, 5 and 6, respectively. They all occur at the N-terminal half of the protein, encompassing the proline-rich region and the SRC domains, upstream from the first PH domain. The 614 G>T mutation, detected as a single observation in patient 25, changes the S205 residue (S205I) located in the proline-rich N-terminal region, a protein-interaction module involved in the localisation of FGD1 protein to the subcortical cytoskeleton and Golgi complex.14 Notably, in the context of proline-rich regions, serine residues frequently flank proline, and are considered to represent, through phosphorylation, a structure for modulation of the protein function.15 The E380A and R443H changes were found in patients 26 and 63, respectively, and subsequently detected in other affected and carrier family members. These two amino-acid changes occur in the conserved Rho/RacGEF domain. In particular, E380 is a residue that resides within the predicted SRC1 and R443 is adjacent to the predicted SRC2 regions.3 Both these residues were conserved at a comparative sequence analysis of the FGD1-related protein.16 All the base substitutions we found were absent in >200 normal X-chromosomes and segregated with the AAS phenotype in the affected family members, thus suggesting that these DNA changes are disease-causing mutations rather than polymorphisms.
Including the mutations reported here, a total of 13 different FGD1 mutations have been reported to cause AAS,3,6,7,8 ‘present report’ consisting of five deletions, two insertions and six missense mutations (Table 1). These mutations are scattered over the whole coding sequence.
The overall mutation detection rate of our study was 19.56%. Therefore, although the sensitivity of the PCR–SSCP method is generally estimated to be around 80%17 and a few mutations may have been missed, the high percentage of patients without mutations may support the hypothesis either of genetic heterogeneity or of a clinical overdiagnoses of this condition.
On the other hand, the mutation detection rate in this study was higher than those reported in two previous studies6,7 (7.69 and 7.40%, respectively). As the clinical criteria were not substantially different from those applied in other studies,6,7 this relatively higher percentage may be explained by the large number of typical patients and by the presence of familial cases with evidence of X-linked inheritance. In three of the four families in which there were more than one affected male, we detected an FGD1 mutation (the families of the probands 26, 58, 90), while in the fourth family, despite a possible X-linked inheritance of the phenotype, no mutations were found. The rate of de novo mutations does not appear particularly high, as illustrated by the documentation of only two sporadic cases (patients 53 and 61: 22.22% of the probands), thus further strengthening the importance of the family evaluation.
As previously reported,6 carrier females often show minor dysmorphic features such as hypertelorism and widow's peak. In the absence of obvious clinical manifestations, we suggest that a minutious physical examination of female family members should be performed as a support for possible X-linked inheritance. This may improve the mutation detection rate, with much more efficient molecular screening and thus optimising the cost-effectiveness of the molecular analysis.
Reviewing the phenotype of the 12 mutated males (nine probands), we found no differences in overall clinical features between patients with truncating mutations and those with missense mutations, nor is there evidence that the location of the FGD1 missense mutations influences the clinical phenotype (Table 2). In addition, on comparing the mutated males with the 37 probands without detected mutations, no global differences were observed with respect to the principal clinical findings. Among mutated patients, the only exception may be the normal stature reported in patient 65, in which the mutation (2530delG) causes a frameshift at the C-terminus of the protein. However, although inter- and intrafamilial variability occurs, mutated patients present with a more full clinical expression of the disease phenotype, with respect to nonmutated patients, in which the entire facio-genito-digital spectrum seems to be displayed less frequently (Table 2). In particular, the association of short stature, hypertelorism and genitourinary abnormalities is the most common presenting feature in both series of patients (mutated and nonmutated). By contrast, palpebral ptosis, syndactyly, joint hyperextension and hernias were documented less frequently in nonmutated patients. These observations, although do not establish a clearcut correlation between phenotype and genotype, may contribute to more strictly clinical diagnostic criteria.
The presence of cognitive problems in AAS patients still remains controversial. It has long been debated whether some degrees of mental retardation and/or behavioural disabilities might be part of the phenotypical spectrum. In the past, a range of mild mental retardation, learning disabilities or attention-deficit disorders have been reported in AAS patients,12 in the absence, however, of a molecular confirmation. The clinical data of the first reported cases of AAS with FGD1 mutations did not support those observations, as mental retardation did not appear to be part of the phenotype of mutated patients.6,7 The recent discovery, however, that the FGD1 mutation P312L is associated to nonsyndromic X-linked mental retardation in a family,8 and the presence of various developmental disabilities and/or behavioural disorders in five of the 12 mutated patients in this study confirms that developmental disabilities may be part of the AAS phenotype. Severe mental retardation, as reported by Lebel et al,8 was not noted in the present group of mutated patients; 12 of our AAS patients had severe mental retardation, and all 12 were found to be negative for FGD1 mutations. Thus, these results suggest that severe mental impairment is not a usual finding in AAS. However, as some aspects of cognitive impairment are recorded among mutated patients, we suggest that mental performance and behavioural phenotype should be considered in the clinical evaluation. Patients 58 and 59 had developmental delay during infancy, although they are of normal intelligence at an older age. Patient 61 had behaviour problems and patients 25 and 50 were mildly mentally retarded.
An interesting finding is that the type of the mutation does not seem to be predictive of whether an affected individual will have some degree of mental impairment or not. The only recurrent mutation we found (528insC) is associated with mild mental impairment in the Belgian family, while the members of the Italian family carrying the same mutation did not show any neurodevelopmental disability. The present study shows that the AAS individuals with mental impairment are only mildly affected, with learning and behavioural disabilities often confined to early childhood. The majority of these children have a good evolution into adulthood and ‘the changing phenotype with age’12 includes an age-related improvement of mental status. However, the risk for a variety of behavioural disturbances and mild learning difficulties appears to be increased in AAS children and specific attention to cognitive and behavioural function is needed in young AAS patients. In conclusion, this study clearly establishes the FGD1 mutational involvement in the X-linked form of AAS and provides new insights into the molecular causation of AAS with a wide spectrum of novel mutations in affected individuals. Finally, this study also confirms that only a minority of the clinically diagnosed patients carries a mutation in the FGD1 gene. The diagnosis of the X-linked AAS needs to be made with care as the spectrum of clinical signs overlaps with that of many different disease entities and, although the phenotype may be impressive, many alternative diagnoses have to be considered.
References
Aarskog D : A familial syndrome of short stature associated with facial dysplasia and genital anomalies. J Pediatr 1970; 77: 856–861.
Scott Jr CI : Unusual facies, joint hypermobility, genital anomaly and short stature. A new dysmorphic syndrome; in Bergsma D, McKusick VA, Konigsmark BW (eds): The clinical delineation of birth defects. Baltimore: Williams and Wilkins, 1971; 10: pp 240–246.
Pasteris NG, Cadle A, Logie LJ et al: Isolation and characterization of the faciogenital dysplasia (Aarskog-Scott syndrome) gene: a putative Rho/Rac guanine nucleotide exchange factor. Cell 1994; 79: 669–678.
Zheng Y, Fischer DJ, Santos MF et al: The faciogenital dysplasia gene product FGD1 functions as a Cdc42Hs-specific guanine-nucleotide exchange factor. J Biol Chem 1996; 271: 33169–33172.
Gorski JL, Estrada L, Hu C, Liu Z : Skeletal-specific expression of Fgd1 during bone formation and skeletal defects in faciogenital dysplasia (FGDY; Aarskog syndrome). Dev Dyn 2000; 218: 573–586.
Orrico A, Galli L, Falciani M et al: A mutation in the pleckstrin homology (PH) domain of the FGD1 gene in an Italian family with faciogenital dysplasia (Aarskog–Scott syndrome). FEBS Lett 2000; 478: 216–220.
Schwartz CE, Gillessen-Kaesbach G, May M et al: Two novel mutations confirm FGD1 is responsible for the Aarskog syndrome. Eur J Hum Genet 2000; 8: 869–874.
Lebel Rr, May M, Pouls S, Lubs H, Stevenson R, Schwartz C : Non-syndromic X-linked mental retardation associated with a missense mutation (P312L) in the FGD1 gene. Clin Genet 2002; 61: 139–145.
Grier RE, Farrington FH, Kendig R, Mamunes P : Autosomal dominant inheritance of the Aarskog syndrome. Am J Med Genet 1983; 15: 39–46.
Teebi AS, Rucquoi JK, Meyn MS : Aarskog syndrome: report of a family with review and discussion of nosology. Am J Med Gene 1993; 46: 501–509.
Porteous MEM, Goudie DR : Aarskog syndrome. J Med Genet 1991; 28: 44–47.
Fryns JP : Aarskog syndrome: the changing phenotype with age. Am J Med Genet 1992; 43: 420–427.
Logie LJ, Porteous MEM : Intelligence and development in Aarskog syndrome. Arch Dis Child 1998; 79: 359–360.
Estrada L, Caron E, Gorski JL : Fgd1, the Cdc42 guanine nucleotide exchange factor responsible for faciogenital dysplasia, is localized to the subcortical actin cytoskeleton and Golgi membrane. Hum Mol Genet 2001; 10: 485–495.
Kay BK, Williamson MP, Sudol M : The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J 2000; 14: 231–241.
Gao J, Estrada L, Cho S, Ellis RE, Gorski JL : The Caenorhabditis elegans homolog of FGD1, the human Cdc42 GEF gene responsible for faciogenital dysplasia, is critical for excretory cell morphogenesis. Hum Mol Genet 2001; 10: 3049–3062.
Sheffield VC, Beck JS, Kwitek AE, Sandstrom DW, Stone EM : The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics 1993; 16: 325–332.
Acknowledgements
We would like to thank all the patients and families who participated in the study and the clinicians who provided blood samples and clinical information. This study was supported by a contribution from Fondazione MPS to V Sorrentino.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orrico, A., Galli, L., Cavaliere, M. et al. Phenotypic and molecular characterisation of the Aarskog–Scott syndrome: a survey of the clinical variability in light of FGD1 mutation analysis in 46 patients. Eur J Hum Genet 12, 16–23 (2004). https://doi.org/10.1038/sj.ejhg.5201081
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejhg.5201081
Keywords
This article is cited by
-
FGD1-related Aarskog–Scott syndrome: Identification of four novel variations and a literature review of clinical and molecular aspects
European Journal of Pediatrics (2024)
-
A novel, putatively null, FGD1 variant leading to Aarskog-Scott syndrome in a family from UAE
BMC Pediatrics (2017)
-
Clinical utility gene card for: Aarskog–Scott Syndrome (faciogenital dysplasia) – update 2015
European Journal of Human Genetics (2015)
-
Aarskog-Scott syndrome: a novel mutation in the FGD1 gene associated with severe craniofacial dysplasia
European Journal of Pediatrics (2014)
-
Clinical utility gene card for: Aarskog–Scott syndrome (faciogenital dysplasia)
European Journal of Human Genetics (2011)