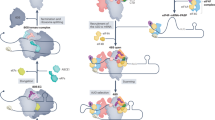
Abstract
Translation initiation in eukaryotes is a complex and highly regulated process requiring the action of at least 12 protein factors. The pathway is distinguished by the formation of a pre-initiation complex that recruits the 5′ end of the mRNA and scans along it to locate the start codon. During the past decade, a combination of genetics, biochemistry and structural studies has begun to illuminate key molecular events in this critical phase of gene expression. Here, we outline our current understanding of eukaryotic translation initiation and discuss important outstanding challenges.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sonenberg, N. & Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136, 731–745 (2009).
Jackson, R.J., Hellen, C.U.T. & Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113–127 (2010).
Hinnebusch, A.G. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 75, 434–467 (2011).
Kolitz, S.E. & Lorsch, J.R. Eukaryotic initiator tRNA: finely tuned and ready for action. FEBS Lett. 584, 396–404 (2010).
Kapp, L.D. & Lorsch, J. GTP-dependent recognition of the methionine moiety on initiator tRNA by translation factor eIF2. J. Mol. Biol. 335, 923–936 (2004).
Naveau, M., Lazennec-Schurdevin, C., Panvert, M., Mechulam, Y. & Schmitt, E. tRNA binding properties of eukaryotic translation initiation factor 2 from Encephalitozoon cuniculi. Biochemistry 49, 8680–8688 (2010).
Shin, B.-S. et al. Initiation factor eIF2γ promotes eIF2–GTP–Met-tRNAiMet ternary complex binding to the 40S ribosome. Nat. Struct. Mol. Biol. 18, 1227–1234 (2011). Provides the first structural information on the position and architecture of the ternary complex on the 40S subunit.
Asano, K., Clayton, J., Shalev, A. & Hinnebusch, A.G. A multifactor complex of eukaryotic initiation factors, eIF1, eIF2, eIF3, eIF5, and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev. 14, 2534–2546 (2000).
Singh, C.R., He, H., Ii, M., Yamamoto, Y. & Asano, K. Efficient incorporation of eukaryotic initiation factor 1 into the multifactor complex is critical for formation of functional ribosomal preinitiation complexes in vivo. J. Biol. Chem. 279, 31910–31920 (2004).
Olsen, D.S. et al. Domains of eIF1A that mediate binding to eIF2, eIF3 and eIF5B and promote ternary complex recruitment in vivo. EMBO J. 22, 193–204 (2003).
Pisarev, A.V. et al. Specific functional interactions of nucleotides at key −3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev. 20, 624–636 (2006).
Asano, K. et al. Multiple roles for the C-terminal domain of eIF5 in translation initiation complex assembly and GTPase activation. EMBO J. 20, 2326–2337 (2001).
Algire, M.A. et al. Development and characterization of a reconstituted yeast translation initiation system. RNA 8, 382–397 (2002).
Majumdar, R., Bandyopadhyay, A. & Maitra, U. Mammalian translation initiation factor eIF1 functions with eIF1A and eIF3 in the formation of a stable 40 40 S preinitiation complex. J. Biol. Chem. 278, 6580–6587 (2003).
Kolupaeva, V.G., Unbehaun, A., Lomakin, I.B., Hellen, C.U.T. & Pestova, T.V. Binding of eukaryotic initiation factor 3 to ribosomal 40S subunits and its role in ribosomal dissociation and anti-association. RNA 11, 470–486 (2005).
Lomakin, I.B. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev. 17, 2786–2797 (2003).
Rabl, J., Leibundgut, M., Ataide, S.F., Haag, A. & Ban, N. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 331, 730–736 (2011). Crystal structure of the Tetrahymena 40S subunit bound to eIF1, suggesting a mechanism for eIF1 release upon full accommodation of the initiator tRNA into the P site.
Yu, Y. et al. Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acids Res. 37, 5167–5182 (2009). Maps the positions of the eIF1A domains on the 40S subunit.
Alone, P.V. & Dever, T.E. Direct binding of translation initiation factor eIF2γ-G domain to its GTPase-activating and GDP-GTP exchange factors eIF5 and eIF2Bɛ. J. Biol. Chem. 281, 12636–12644 (2006).
Paulin, F.E., Campbell, L.E., O'Brien, K., Loughlin, J. & Proud, C.G. Eukaryotic translation initiation factor 5 (eIF5) acts as a classical GTPase-activator protein. Curr. Biol. 11, 55–59 (2001).
Das, S., Ghosh, R. & Maitra, U. Eukaryotic translation initiation factor 5 functions as a GTPase-activating protein. J. Biol. Chem. 276, 6720–6726 (2001).
Asano, K., Krishnamoorthy, T., Phan, L., Pavitt, G.D. & Hinnebusch, A.G. Conserved bipartite motifs in yeast eIF5 and eIF2Bɛ, GTPase-activating and GDP-GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J. 18, 1673–1688 (1999).
Yamamoto, Y. et al. The eukaryotic initiation factor (eIF) 5 HEAT domain mediates multifactor assembly and scanning with distinct interfaces to eIF1, eIF2, eIF3, and eIF4G. Proc. Natl. Acad. Sci. USA 102, 16164–16169 (2005).
Siridechadilok, B., Fraser, C., Hall, R. & Doudna, J. Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science 310, 1513–1515 (2005).
Pisarev, A.V., Kolupaeva, V.G., Yusupov, M.M., Hellen, C.U.T. & Pestova, T.V. Ribosomal position and contacts of mRNA in eukaryotic translation initiation complexes. EMBO J. 27, 1609–1621 (2008). Provides structural information on the relative positions of eIF3 and mRNA on the 40S subunit.
Valášek, L. et al. The yeast eIF3 subunits TIF32/a, NIP1/c, and eIF5 make critical connections with the 40S ribosome in vivo. Genes Dev. 17, 786–799 (2003).
Chiu, W.-L. et al. The C-terminal region of eukaryotic translation initiation factor 3a (eIF3a) promotes mRNA recruitment, scanning, and, together with eIF3j and the eIF3b RNA recognition motif, selection of AUG start codons. Mol. Cell. Biol. 30, 4415–4434 (2010).
Sokabe, M., Fraser, C.S. & Hershey, J.W.B. The human translation initiation multi-factor complex promotes methionyl-tRNAi binding to the 40S ribosomal subunit. Nucleic Acids Res. 40, 905–913 (2012).
Valášek, L., Nielsen, K.H. & Hinnebusch, A.G. Direct eIF2-eIF3 contact in the multifactor complex is important for translation initiation in vivo. EMBO J. 21, 5886–5898 (2002).
Nielsen, K.H. et al. Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J. 23, 1166–1177 (2004).
Valášek, L., Nielsen, K.H., Zhang, F., Fekete, C.A. & Hinnebusch, A.G. Interactions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol. Cell. Biol. 24, 9437–9455 (2004).
Singh, C.R. et al. Eukaryotic translation initiation factor 5 is critical for integrity of the scanning preinitiation complex and accurate control of GCN4 translation. Mol. Cell. Biol. 25, 5480–5491 (2005).
Jivotovskaya, A.V., Valášek, L., Hinnebusch, A.G. & Nielsen, K.H. Eukaryotic translation initiation factor 3 (eIF3) and eIF2 can promote mRNA binding to 40S subunits independently of eIF4G in yeast. Mol. Cell. Biol. 26, 1355–1372 (2006). Provides evidence for the central role of eIF3 in mRNA recruitment in vivo.
Hasek, J. et al. Rpg1p, the subunit of the Saccharomyces cerevisiae eIF3 core complex, is a microtubule-interacting protein. Cell Motil. Cytoskeleton 45, 235–246 (2000).
Palecek, J., Hasek, J. & Ruis, H. Rpg1p/Tif32p, a subunit of translation initiation factor 3, interacts with actin-associated protein Sla2p. Biochem. Biophys. Res. Commun. 282, 1244–1250 (2001).
Pincheira, R., Chen, Q., Huang, Z. & Zhang, J.T. Two subcellular localizations of eIF3 p170 and its interaction with membrane-bound microfilaments: implications for alternative functions of p170. Eur. J. Cell Biol. 80, 410–418 (2001).
Maag, D. Communication between eukaryotic translation initiation factors 1 and 1A on the yeast small ribosomal subunit. J. Mol. Biol. 330, 917–924 (2003).
Pestova, T.V., Borukhov, S.I. & Hellen, C.U.T. Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature 394, 854–859 (1998).
Passmore, L.A. et al. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol. Cell 26, 41–50 (2007). Cryo-EM reconstructions highlighting the role of eIF1 in stabilizing the open state of the 40S subunit.
Maag, D., Fekete, C., Gryczynski, Z. & Lorsch, J. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol. Cell 17, 265–275 (2005).Demonstrates that eIF1 is released from the PIC upon start codon recognition.
Saini, A.K., Nanda, J.S., Lorsch, J.R. & Hinnebusch, A.G. Regulatory elements in eIF1A control the fidelity of start codon selection by modulating tRNAiMet binding to the ribosome. Genes Dev. 24, 97–110 (2010). Maps the regions of the eIF1A CTT and NTT responsible for their opposing roles in start codon recognition.
Fekete, C.A. et al. N- and C-terminal residues of eIF1A have opposing effects on the fidelity of start codon selection. EMBO J. 26, 1602–1614 (2007).
Chaudhuri, J., Chowdhury, D. & Maitra, U. Distinct functions of eukaryotic translation initiation factors eIF1A and eIF3 in the formation of the 40 S ribosomal preinitiation complex. J. Biol. Chem. 274, 17975–17980 (1999).
Uchida, N., Hoshino, S.-I., Imataka, H., Sonenberg, N. & Katada, T. A novel role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in Cap/Poly(A)-dependent translation. J. Biol. Chem. 277, 50286–50292 (2002).
Park, E.-H. et al. Multiple elements in the eIF4G1 N-terminus promote assembly of eIF4G1•PABP mRNPs in vivo. EMBO J. 30, 302–316 (2011).
Kaye, N.M., Emmett, K.J., Merrick, W.C. & Jankowsky, E. Intrinsic RNA binding by the eukaryotic initiation factor 4F depends on a minimal RNA length but not on the m7G cap. J. Biol. Chem. 284, 17742–17750 (2009).
Mitchell, S.F. et al. The 5′-7-methylguanosine cap on eukaryotic mRNAs serves both to stimulate canonical translation initiation and to block an alternative pathway. Mol. Cell 39, 950–962 (2010). Demonstrates that eIF3 is critical for mRNA recruitment in vitro and identifies a new role for the 5′ cap in blocking the formation of aberrant 43S–mRNA complexes.
Yanagiya, A. et al. Requirement of RNA binding of mammalian eukaryotic translation initiation factor 4GI (eIF4GI) for efficient interaction of eIF4E with the mRNA cap. Mol. Cell. Biol. 29, 1661–1669 (2009).
Tarun, S.Z., Wells, S., Deardorff, J. & Sachs, A. Translation initiation factor eIF4G mediates in vitro poly(A) tail-dependent translation. Proc. Natl. Acad. Sci. USA 94, 9046–9051 (1997).
Kahvejian, A., Svitkin, Y.V., Sukarieh, R., M'Boutchou, M.-N. & Sonenberg, N. Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor, which acts via multiple mechanisms. Genes Dev. 19, 104–113 (2005).
Hinton, T.M., Coldwell, M.J., Carpenter, G.A., Morley, S.J. & Pain, V.M. Functional analysis of individual binding activities of the scaffold protein eIF4G. J. Biol. Chem. 282, 1695–1708 (2007).
Svitkin, Y.V. et al. General RNA-binding proteins have a function in poly(A)-binding protein-dependent translation. EMBO J. 28, 58–68 (2009). Provides evidence that the effects of PABP and the poly(A) tail are most pronounced under conditions of strong competition for mRNA binding.
Ramírez-Valle, F., Braunstein, S., Zavadil, J., Formenti, S.C. & Schneider, R.J. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J. Cell Biol. 181, 293–307 (2008).
Park, E.-H., Zhang, F., Warringer, J., Sunnerhagen, P. & Hinnebusch, A.G. Depletion of eIF4G from yeast cells narrows the range of translational efficiencies genome-wide. BMC Genomics 12, 68 (2011).
Mayberry, L.K., Allen, M.L., Dennis, M.D. & Browning, K.S. Evidence for variation in the optimal translation initiation complex: plant eIF4B, eIF4F, and eIF(iso)4F differentially promote translation of mRNAs. Plant Physiol. 150, 1844–1854 (2009).
Lorsch, J.R. & Herschlag, D. The DEAD box protein eIF4A. 1. A minimal kinetic and thermodynamic framework reveals coupled binding of RNA and nucleotide. Biochemistry 37, 2180–2193 (1998).
Sengoku, T., Nureki, O., Nakamura, A., Kobayashi, S. & Yokoyama, S. Structural basis for RNA unwinding by the DEAD-box protein Drosophila Vasa. Cell 125, 287–300 (2006).
Liu, F., Putnam, A. & Jankowsky, E. ATP hydrolysis is required for DEAD-box protein recycling but not for duplex unwinding. Proc. Natl. Acad. Sci. USA 105, 20209–20214 (2008).
Oberer, M., Marintchev, A. & Wagner, G. Structural basis for the enhancement of eIF4A helicase activity by eIF4G. Genes Dev. 19, 2212–2223 (2005).
Marintchev, A. et al. Topology and regulation of the human eIF4A/4G/4H helicase complex in translation initiation. Cell 136, 447–460 (2009).
Hilbert, M., Kebbel, F., Gubaev, A. & Klostermeier, D. eIF4G stimulates the activity of the DEAD box protein eIF4A by a conformational guidance mechanism. Nucleic Acids Res. 39, 2260–2270 (2011).
Rogers, G.W., Richter, N.J., Lima, W.F. & Merrick, W.C. Modulation of the helicase activity of eIF4A by eIF4B, eIF4H, and eIF4F. J. Biol. Chem. 276, 30914–30922 (2001).
Rozovsky, N., Butterworth, A.C. & Moore, M.J. Interactions between eIF4AI and its accessory factors eIF4B and eIF4H. RNA 14, 2136–2148 (2008).
Bi, X., Ren, J. & Goss, D.J. Wheat germ translation initiation factor eIF4B affects eIF4A and eIFiso4F helicase activity by increasing the ATP binding affinity of eIF4A. Biochemistry 39, 5758–5765 (2000).
Cheng, S., Sultana, S., Goss, D.J. & Gallie, D.R. Translation initiation factor 4B homodimerization, RNA binding, and interaction with Poly(A)-binding protein are enhanced by zinc. J. Biol. Chem. 283, 36140–36153 (2008).
Özeş, A.R., Feoktistova, K., Avanzino, B.C. & Fraser, C.S. Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B. J. Mol. Biol. 412, 674–687 (2011).
Nielsen, K.H. et al. Synergistic activation of eIF4A by eIF4B and eIF4G. Nucleic Acids Res. 39, 2678–2689 (2011).
Altmann, M., Wittmer, B., Methot, N., Sonenberg, N. & Trachsel, H. The Saccharomyces cerevisiae translation initiation factor Tif3 and its mammalian homologue, eIF-4B, have RNA annealing activity. EMBO J. 14, 3820–3827 (1995).
Niederberger, N., Trachsel, H. & Altmann, M. The RNA recognition motif of yeast translation initiation factor Tif3/eIF4B is required but not sufficient for RNA strand-exchange and translational activity. RNA 4, 1259–1267 (1998).
LeFebvre, A.K. et al. Translation initiation factor eIF4G–1 binds to eIF3 through the eIF3e subunit. J. Biol. Chem. 281, 22917–22932 (2006).
Méthot, N., Song, M.S. & Sonenberg, N. A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Mol. Cell. Biol. 16, 5328–5334 (1996).
Dmitriev, S.E., Terenin, I., Dunaevsky, Y., Merrick, W. & Shatsky, I. Assembly of 48S translation initiation complexes from purified components with mRNAs that have some base pairing within their 5′ untranslated regions. Mol. Cell. Biol. 23, 8925–8933 (2003).
Hinnebusch, A.G. eIF3: a versatile scaffold for translation initiation complexes. Trends Biochem. Sci. 31, 553–562 (2006).
Nielsen, K.H., Valasek, L., Sykes, C., Jivotovskaya, A. & Hinnebusch, A.G. Interaction of the RNP1 motif in PRT1 with HCR1 promotes 40S binding of eukaryotic initiation factor 3 in yeast. Mol. Cell. Biol. 26, 2984–2998 (2006).
Unbehaun, A., Borukhov, S.I., Hellen, C.U.T. & Pestova, T.V. Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon-anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev. 18, 3078–3093 (2004).
Cuchalová, L. et al. The RNA recognition motif of eukaryotic translation initiation factor 3g (eIF3g) is required for resumption of scanning of posttermination ribosomes for reinitiation on GCN4 and together with eIF3i stimulates linear scanning. Mol. Cell. Biol. 30, 4671–4686 (2010).
Elantak, L. et al. The indispensable N-terminal half of eIF3j/HCR1 cooperates with its structurally conserved binding partner eIF3b/PRT1-RRM and with eIF1A in stringent AUG selection. J. Mol. Biol. 396, 1097–1116 (2010).
Mignone, F., Gissi, C., Liuni, S. & Pesole, G. Untranslated regions of mRNAs. Genome Biol. 3, reviews0004.1–reviews0004.10 (2002).
Ivanov, I.P., Loughran, G., Sachs, M.S. & Atkins, J.F. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1). Proc. Natl. Acad. Sci. USA 107, 18056–18060 (2010).
Martin-Marcos, P., Cheung, Y.-N. & Hinnebusch, A.G. Functional elements in initiation factors 1, 1A, and 2β discriminate against poor AUG context and non-AUG start codons. Mol. Cell. Biol. 31, 4814–4831 (2011).
Berthelot, K., Muldoon, M., Rajkowitsch, L., Hughes, J. & McCarthy, J.E.G. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 51, 987–1001 (2004).
Vassilenko, K.S., Alekhina, O.M., Dmitriev, S.E., Shatsky, I.N. & Spirin, A.S. Unidirectional constant rate motion of the ribosomal scanning particle during eukaryotic translation initiation. Nucleic Acids Res. 39, 5555–5567 (2011).
Pestova, T.V. & Kolupaeva, V. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 16, 2906–2922 (2002).
Prévôt, D. et al. Characterization of a novel RNA-binding region of eIF4GI critical for ribosomal scanning. EMBO J. 22, 1909–1921 (2003).
Watanabe, R. et al. The eukaryotic initiation factor (eIF) 4G HEAT domain promotes translation re-initiation in yeast both dependent on and independent of eIF4A mRNA helicase. J. Biol. Chem. 285, 21922–21933 (2010).
Lindqvist, L., Imataka, H. & Pelletier, J. Cap-dependent eukaryotic initiation factor-mRNA interactions probed by cross-linking. RNA 14, 960–969 (2008).
von der Haar, T. & McCarthy, J.E. Intracellular translation initiation factor levels in Saccharomyces cerevisiae and their role in cap-complex function. Mol. Microbiol. 46, 531–544 (2002).
Pisareva, V.P., Pisarev, A.V., Komar, A.A., Hellen, C.U.T. & Pestova, T.V. Translation initiation on mammalian mRNAs with structured 5′UTRs requires DExH-box protein DHX29. Cell 135, 1237–1250 (2008). Demonstrates that helicases besides eIF4A are required for recruitment and scanning of mRNAs containing highly stable, secondary structure elements.
Abaeva, I.S., Marintchev, A., Pisareva, V.P., Hellen, C.U.T. & Pestova, T.V. Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning. EMBO J. 30, 115–129 (2011).
Spirin, A.S. How does a scanning ribosomal particle move along the 5′-untranslated region of eukaryotic mRNA? Brownian Ratchet model. Biochemistry 48, 10688–10692 (2009).
Rajagopal, V., Park, E.-H., Hinnebusch, A.G. & Lorsch, J.R. Specific domains in yeast eIF4G strongly bias the RNA unwinding activity of the eIF4F complex towards duplexes with 5′-overhangs. J. Biol. Chem. published online, doi:10.1074/jbc.M112.347278 (30 March 2012).
Algire, M.A., Maag, D. & Lorsch, J.R. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20, 251–262 (2005).
Cheung, Y.-N. et al. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 21, 1217–1230 (2007). Demonstrates the key role of eIF1 in maintaining start codon fidelity in vivo.
Nanda, J.S. et al. eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J. Mol. Biol. 394, 268–285 (2009).
Reibarkh, M. et al. Eukaryotic initiation factor (eIF) 1 carries two distinct eIF5-binding faces important for multifactor assembly and AUG selection. J. Biol. Chem. 283, 1094–1103 (2008).
Loughran, G., Sachs, M.S., Atkins, J.F. & Ivanov, I.P. Stringency of start codon selection modulates autoregulation of translation initiation factor eIF5. Nucleic Acids Res. 40, 2898–2906 (2012).
Maag, D., Algire, M.A. & Lorsch, J.R. Communication between eukaryotic translation initiation factors 5 and 1A within the ribosomal pre-initiation complex plays a role in start site selection. J. Mol. Biol. 356, 724–737 (2006).
Herrmannová, A. et al. Structural analysis of an eIF3 subcomplex reveals conserved interactions required for a stable and proper translation pre-initiation complex assembly. Nucleic Acids Res. 40, 2294–2311 (2012).
Pestova, T.V. et al. The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 403, 332–335 (2000).
Acker, M.G., Shin, B.-S., Dever, T.E. & Lorsch, J.R. Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining. J. Biol. Chem. 281, 8469–8475 (2006).
Acker, M.G. et al. Kinetic analysis of late steps of eukaryotic translation initiation. J. Mol. Biol. 385, 491–506 (2009).
Shin, B.S. et al. Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity. Cell 111, 1015–1025 (2002).
Marintchev, A., Kolupaeva, V., Pestova, T. & Wagner, G. Mapping the binding interface between human eukaryotic initiation factors 1A and 5B: a new interaction between old partners. Proc. Natl. Acad. Sci. USA 100, 1535–1540 (2003).
Szamecz, B. et al. eIF3a cooperates with sequences 5′ of uORF1 to promote resumption of scanning by post-termination ribosomes for reinitiation on GCN4 mRNA. Genes Dev. 22, 2414–2425 (2008). Identifies a role for eIF3 in scanning and reinitiation in vivo.
Pöyry, T.A.A., Kaminski, A., Connell, E.J., Fraser, C.S. & Jackson, R.J. The mechanism of an exceptional case of reinitiation after translation of a long ORF reveals why such events do not generally occur in mammalian mRNA translation. Genes Dev. 21, 3149–3162 (2007).
Ben-Shem, A. et al. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science 334, 1524–1529 (2011).
You, T., Coghill, G.M. & Brown, A.J.P. A quantitative model for mRNA translation in Saccharomyces cerevisiae. Yeast 27, 785–800 (2010).
You, T., Stansfield, I., Romano, M.C., Brown, A.J.P. & Coghill, G.M. Analysing GCN4 translational control in yeast by stochastic chemical kinetics modelling and simulation. BMC Syst. Biol. 5, 131 (2011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Aitken, C., Lorsch, J. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol 19, 568–576 (2012). https://doi.org/10.1038/nsmb.2303
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2303
This article is cited by
-
The molecular basis of translation initiation and its regulation in eukaryotes
Nature Reviews Molecular Cell Biology (2024)
-
Oligonucleotide mapping via mass spectrometry to enable comprehensive primary structure characterization of an mRNA vaccine against SARS-CoV-2
Scientific Reports (2023)
-
A novel computational method enables RNA editome profiling during human hematopoiesis from scRNA-seq data
Scientific Reports (2023)
-
Translation regulatory factor BZW1 regulates preimplantation embryo development and compaction by restricting global non-AUG Initiation
Nature Communications (2022)
-
CircRNAs and their regulatory roles in cancers
Molecular Medicine (2021)