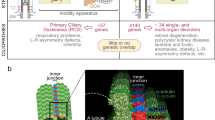
Key Points
-
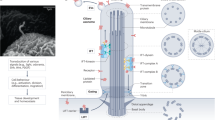
Cilia are complex sensory and motile organelles found on almost all cells of the body. The complexity of the cilium raises the question of how it is built in an orderly fashion.
-
Ciliary assembly proceeds in a stepwise manner: centrioles form basal bodies, dock on the cortex and induce outgrowth of the cilium. Assembly also involves protein-trafficking from the cytoplasm to the base of the cilium and selective import of ciliary proteins through a channel that may be analogous to the nuclear pore complex.
-
Sustained growth of cilia requires active transport, which is provided by the intraflagellar transport (IFT) system.
-
Assembly of cilia is a function of cell cycle stage and is tightly regulated to control the length of the final structure.
-
Ciliary length seems to result from a continuous steady-state balance of assembly and disassembly, with the inherent length-dependence of IFT-mediated transport leading to a length-dependent assembly rate.
Abstract
The cilium is a complex organelle, the assembly of which requires the coordination of motor-driven intraflagellar transport (IFT), membrane trafficking and selective import of cilium-specific proteins through a barrier at the ciliary transition zone. Recent findings provide insights into how cilia assemble and disassemble in synchrony with the cell cycle and how the balance of ciliary assembly and disassembly determines the steady-state ciliary length, with the inherent length-dependence of IFT rendering the ciliary assembly rate a decreasing function of length. As cilia are important in sensing and processing developmental signals and directing the flow of fluids such as mucus, defects in ciliogenesis and length control are likely to underlie a range of cilium-related human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Singla, V. & Reiter, J. F. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science 313, 629–633 (2006).
Berbari, N. F., O'Connor, A. K., Haycraft, C. J. & Yoder, B. K. The primary cilium as a complex signaling center. Curr. Biol. 19, R526–R535 (2009).
Gherman, A., Davis, E. E. & Katsanis, N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nature Genet. 38, 961–962 (2006).
Gaertig, J. & Wloga, D. Ciliary tubulin and its post-translational modifications. Curr. Top. Dev. Biol. 85, 83–113 (2008).
Thazhath, R. et al. Cell context-specific effects of the β-tubulin glycylation domain on assembly and size of microtubular organelles. Mol. Biol. Cell 15, 4136–4147 (2004).
Pathak, N., Obara, T., Mangos, S., Liu, Y. & Drummond, I. A. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol. Biol. Cell 18, 4353–4364 (2007). Demonstrates that a protein required for tubulin modification also plays a part in building the outer doublet microtubule structure.
Wloga, D. et al. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell 16, 867–876 (2009).
Kubo, T., Yanagisawa, H., Yagi, T., Hirono, M. & Kamiya, R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr. Biol. 20, 441–445 (2010).
Suryavanshi, S. et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr. Biol. 20, 435–440 (2010).
Ikegami, K., Sato, S., Nakamura, K., Ostrowski, L. E. & Setou, M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc. Natl Acad. Sci. USA 107, 10490–10495 (2010).
Steffen, W. & Linck, R. W. Evidence for tektins in centrioles and axonemal microtubules. Proc. Natl Acad. Sci. USA 85, 2643–2647 (1988).
Linck, R. W. & Norrander, J. M. Protofilament ribbon compartments of ciliary and flagellar microtubules. Protist 154, 299–311 (2003).
Nojima, D., Linck, R. W. & Egelman, E. H. At least one of the protofilaments in flagellar microtubules is not composed of tubulin. Curr. Biol. 5, 158–167 (1995).
Sui, H. & Downing, K. H. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442, 475–478 (2006).
Nicastro, D. et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science 313, 944–948 (2006).
Sorokin, S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J. Cell Biol. 15, 363–377 (1962).
Sorokin, S. P. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J. Cell Sci. 3, 207–230 (1968). Classic ultrastructural description of the individual steps that lead to the production of cilia from basal bodies.
Molla-Herman, A. et al. The ciliary pocket: an endocytic membrane domain at the base of primary and motile cilia. J. Cell Sci. 123, 1785–1795 (2010).
Rosenbaum, J. L. & Child., F. M. Flagellar regeneration in protozoan flagellates. J. Cell Biol. 34, 345–364 (1967).
Sinden, R. E., Canning, E. U. & Spain, B. Gametogenesis and fertilization in Plasmodium yoelii nigeriensis: a transmission electron microscope study. Proc. R. Soc. Lond. B 193, 55–76 (1976).
Marshall, W. F. & Rosenbaum, J. L. Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J. Cell Biol. 155, 405–414 (2001).
Stephens, R. E. Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol. Biol. Cell 8, 2187–2198 (1997). A dramatic demonstration that microtubules in the ciliary axoneme are not static but undergo continuous turnover.
Song, L. & Dentler, W. L. Flagellar protein dynamics in Chlamydomonas. J. Biol. Chem. 276, 29754–29763 (2001).
Blaineau, C. et al. A novel microtubule-depolymerizing kinesin involved in length control of a eukaryotic flagellum. Curr. Biol. 17, 778–782 (2007).
Dawson, S. C. et al. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryotic Cell 6, 2354–2364 (2007).
Piao, T. et al. A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc. Natl Acad. Sci. USA 106, 4713–4718 (2009).
Pedersen, L. B. & Rosenbaum, J. L. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Curr. Top. Dev. Biol. 85, 23–61 (2008).
Hao, L. & Scholey, J. M. Intraflagellar transport at a glance. J. Cell Sci. 122, 889–892 (2009).
Kozminski, K. G., Johnson, K. A., Forscher, P. & Rosenbaum, J. L. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl Acad. Sci. USA 90, 5519–5523 (1993). The first reported observation of intraflagellar transport detected by differential interference contrast microscopy analysis of C. reinhardtii flagella.
Kozminski, K. G., Beech, P. L. & Rosenbaum, J. L. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J. Cell Biol. 131, 1517–1527 (1995).
Pigino, G. et al. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 187, 135–148 (2009).
Rosenbaum, J. L. & Witman, G. B. Intraflagellar transport. Nature Rev. Mol. Cell Biol. 3, 813–825 (2002).
Cole, D. G. et al. Novel heterotrimeric kinesin-related protein purified from sea urchin eggs. Nature 366, 268–270 (1993).
Morris, R. L. & Scholey, J. M. Heterotrimeric kinesin-II is required for the assembly of motile 9+2 ciliary axonemes on sea urchin embryos. J. Cell Biol. 138, 1009–1022 (1997).
Nonaka, S. et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell 95, 829–837 (1998).
Mueller, J., Perrone, C. A., Bower, R., Cole, D. G. & Porter, M. E. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell 16, 1341–1354 (2005).
Snow, J. J. et al. Two anterograde intraflagellar transport motors cooperate to build sensory cilia on C. elegans neurons. Nature Cell Biol. 6, 1109–1113 (2004).
Pan, X. et al. Mechanism of transport of IFT particles in C. elegans cilia by the concerted action of kinesin-II and OSM-3 motors. J. Cell Biol. 174, 1035–1045 (2006).
Evans, J. E. et al. Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in Caenorhabditis elegans. J. Cell Biol. 172, 663–669 (2006).
Mukhopadhyay, S. et al. Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in C. elegans. EMBO J. 26, 2966–2980 (2007). By analyzing the structure of different classes of cilia in worms carrying IFT mutations, the authors show that different components of the IFT machinery are differentially required for building different cilia.
Pazour, G. J., Wilkerson, C. G. & Witman, G. B. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J. Cell Biol. 141, 979–992 (1998).
Pazour, G. J., Dickert, B. L. & Witman, G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 (1999).
Porter, M. E., Bower, R., Knott, J. A., Byrd, P. & Dentler, W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol. Biol. Cell 10, 693–712 (1999).
Signor, D. et al. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J. Cell Biol. 147, 519–530 (1999).
Hou, Y., Pazour, G. J. & Witman, G. B. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol. Biol. Cell 15, 4382–4394 (2004).
Huangfu, D. & Anderson, K. V. Cilia and Hedgehog responsiveness in the mouse. Proc. Natl Acad. Sci. USA 102, 11325–11330 (2005).
May, S. R. et al. Loss of the retrograde motor for IFT disrupts localization of Smo to cilia and prevents the expression of both activator and repressor functions of Gli. Dev. Biol. 287, 378–389 (2005).
Piperno, G. & Mead, K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl Acad. Sci. USA 94, 4457–4462 (1997).
Cole, D. G. et al. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 141, 993–1008 (1998). The first biochemical analysis of IFT components, exploiting the advantages of C. reinhardtii for both biochemistry and genetics, and leading to the realization that proteins involved in IFT are related to polycystic kidney disease gene products.
Cole, D. G. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic 4, 435–442 (2003).
Avidor-Reiss, T. et al. Decoding cilia function: defining specialized genes required for compartmentalized cilia biogenesis. Cell 117, 527–539 (2004).
Wang, Z., Fan, Z., Williamson, S. M. & Qin, H. Intraflagellar transport (IFT) protein IFT25 is a phosphoprotein component of IFT complex B and physically interacts with IFT27 in Chlamydomonas. PLoS ONE 4, e5384 (2009).
Follit, J. A., Xu, F., Keady, B. T. & Pazour, G. J. Characterization of mouse IFT complex B. Cell Motil. Cytoskeleton 66, 457–468 (2009).
Lechtreck, K., Luro, S., Awata, J. & Witman, G. B. HA-tagging of putative flagellar proteins in Chlamydomonas reinhardtii identifies a novel protein of intraflagellar transport complex B. Cell Motil. Cytoskeleton 66, 469–482 (2009).
Qin, H., Wang, Z., Diener, D. & Rosenbaum, J. Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr. Biol. 17, 193–202 (2007). Reports the discovery of a key IFT protein that is a member of a protein family involved in many aspects of membrane trafficking.
Schafer, J. C. et al. IFTA-2 is a conserved cilia protein involved in pathways regulating longevity and dauer formation in Caenorhabditis elegans. J. Cell Sci. 119, 4088–4100 (2006).
Ou, G., Blacque, O. E., Snow, J. J., Leroux, M. R. & Scholey, J. M. Functional coordination of intraflagellar transport motors. Nature 436, 583–587 (2005).
Fan, Z. et al. Chlamydomonas IFT70/CrDYF-1 is a core component of IFT particle complex B and is required for flagellar assembly. Mol. Biol. Cell 21, 2696–2706 (2010).
Dave, D., Wloga, D., Sharma, N. & Gaertig, J. DYF-1 is required for assembly of the axoneme in Tetrahymena thermophila. Eukaryotic Cell 8, 1397–1406 (2009).
Fujiwara, M., Ishihara, T. & Katsura, I. A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development 126, 4839–4848 (1999).
Pazour, G. J. et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 151, 709–718 (2000).
Brazelton, W. J., Amundsen, C. D., Silflow, C. D. & Lefebvre, P. A. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 11, 1591–1594 (2001).
Deane, J. A., Cole, D. G., Seeley, E. S., Diener, D. R. & Rosenbaum, J. L. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr. Biol. 11, 1586–1590 (2001).
Haycraft, C. J., Schafer, J. C., Zhang, Q., Taulman, P. D. & Yoder, B. K. Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp. Cell Res. 284, 251–263 (2003).
Huangfu, D. et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003). The first report linking cilia with Hedgehog signalling.
Sun, Z. et al. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131, 4085–4093 (2004). Describes the use of a forward genetic screen in zebrafish to find candidate cystic kidney disease genes, which revealed a number of IFT protein-encoding genes.
Follit, J. A., Tuft, R. A., Fogarty, K. E. & Pazour, G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 17, 3781–3792 (2006).
Iomini, C., Babaev-Khaimov, V., Sassaroli, M. & Piperno, G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 153, 13–24 (2001).
Efimenko, E. et al. Caenorhabditis elegans DYF-2, an orthologue of human WDR19, is a component of the intraflagellar transport machinery in sensory cilia. Mol. Biol. Cell 17, 4801–4811 (2006).
Tsao, C. & Gorovsky, M. A. Tetrahymena IFT122A is not essential for cilia assembly but plays a role in returning IFT proteins from the ciliary tip to the cell body. J. Cell Sci. 121, 428–436 (2008).
Absalon, S. et al. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol. Biol. Cell 19, 929–944 (2008).
Tran, P. V. et al. THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nature Genet. 40, 403–410 (2008).
Iomini, C., Li, L., Esparza, J. M. & Dutcher, S. K. Retrograde intraflagellar transport mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics 183, 885–896 (2009).
Piperno, G. et al. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 143, 1591–1601 (1998).
Murayama, T., Toh, Y., Ohshima, Y. & Koga, M. The dyf-3 gene encodes a novel protein required for sensory cilium formation in Caenorhabditis elegans. J. Mol. Biol. 346, 677–687 (2005).
Blacque, O. E. et al. Functional genomics of the cilium, a sensory organelle. Curr. Biol. 15, 935–941 (2005).
Ou, G., Qin, H., Rosenbaum, J. L. & Scholey, J. M. The PKD protein qilin undergoes intraflagellar transport. Curr. Biol. 15, R410–R411 (2005).
Ou, G. et al. Sensory ciliogenesis in Caenorhabditis elegans: assignment of IFT components into distinct modules based on transport and phenotypic profiles. Mol. Biol. Cell 18, 1554–1569 (2007).
Mukhopadhyay, S. et al. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 24, 2180–2193 (2010).
Blacque, O. E. et al. Loss of C. elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes Dev. 18, 1630–1642 (2004).
Nachury, M. V. et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129, 1201–1213 (2007). Biochemical- and RNAi-based analysis of the BBSome, suggesting a role for it in ciliary assembly via regulation of membrane trafficking.
Mykytyn, K. et al. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc. Natl Acad. Sci. USA 101, 8664–8669 (2004).
Nishimura, D. Y. et al. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc. Natl Acad. Sci. USA 101, 16588–16593 (2004).
Fath, M. A. et al. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Hum. Mol. Genet. 14, 1109–1118 (2005).
Davis, R. E. et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc. Natl Acad. Sci. USA 104, 19422–19427 (2007).
Loktev, A. V. et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev. Cell 15, 854–865 (2008).
Tan, P. L. et al. Loss of Bardet Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proc. Natl Acad. Sci. USA 104, 17524–17529 (2007).
Berbari, N. F., Lewis, J. S., Bishop, G. A., Askwith, C. C. & Mykytyn, K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc. Natl Acad. Sci. USA 105, 4242–4246 (2008).
Lechtreck, K. et al. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J. Cell Biol. 187, 1117–1132 (2009).
Qin, H., Diener, D. R., Geimer, S., Cole, D. G. & Rosenbaum, J. L. Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J. Cell Biol. 164, 255–266 (2004). Presents the first direct evidence that IFT particles can actually bind cargo, by showing that many flagellar proteins co-immunoprecipitate with IFT particles isolated from C. reinhardtii flagella.
Marshall, W. F., Qin, H., Rodrigo Brenni, M. & Rosenbaum, J. L. Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol. Biol. Cell 16, 270–278 (2005). Describes several experiments to test the balance-point model for length control, including testing predictions concerning the variation of flagellar length with number.
Qin, H. et al. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr. Biol. 15, 1695–1699 (2005). Demonstrates that IFT can move channels in the ciliary membrane, suggesting a possible influence of IFT on signalling activity.
Huang, K. et al. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501–514 (2007).
Hou, Y. et al. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 176, 653–665 (2007).
Ahmed, N. T., Gao, C., Lucker, B. F., Cole, D. G. & Mitchell, D. R. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J. Cell Biol. 183, 313–322 (2008). Provides the first evidence that IFT particles may use adaptor proteins to carry specific subsets of cargo.
Piperno, G., Mead, K. & Henderson, S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1FLA10 to reach the distal part of flagella in Chlamydomonas. J. Cell Biol. 133, 371–379 (1996).
Gao, C., Wang, G., Amack, J. D. & Mitchell, D. R. Oda16/Wdr69 is essential for axonemal dynein assembly and ciliary motility during zebrafish embryogenesis. Dev. Dyn. 239, 2190–2197 (2010).
Omori, Y. et al. elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nature Cell Biol. 10, 437–444 (2008).
Li, C. et al. An essential role for DYF-11/MIP-T3 in assembling functional intraflagellar transport complexes. PLoS Genet. 4, e1000044 (2008).
Yoshimura, S., Egerer, J., Fuchs, E., Haas, A. K. & Barr, F. A. Functional dissection of Rab GTPases involved in primary cilium formation. J. Cell Biol. 178, 363–369 (2007).
Jin, H. et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141, 1208–1219 (2010).
Jékely, G. & Arendt, D. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 28, 191–198 (2006).
Finetti, F. et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nature Cell Biol. 11, 1332–1339 (2009).
Sedmak, T. & Wolfrum, U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. J. Cell Biol. 189, 171–186 (2010).
Craige, B. et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J. Cell Biol. 190, 927–940 (2010). Identifies a key regulator of protein entry through the ciliary transition zone.
Hu, Q. et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329, 436–439 (2010). Presents evidence that septins, which form barriers to the lateral diffusion of proteins through the cell membrane during cell division, perform a similar function at the base of cilia.
Dishinger, J. F. et al. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-β2 and RanGTP. Nature Cell Biol. 12, 703–710 (2010). Suggests that ciliary import may be regulated by some of the same proteins that mediate vectorial transport through the nuclear pore complex.
Geng, L. et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J. Cell. Sci. 119, 1383–1395 (2006).
Jenkins, P. M. et al. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Curr. Biol. 16, 1211–1216 (2006).
Mazelova, J. et al. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J. 28, 183–192 (2009).
Tao, B. et al. Cystin localizes to primary cilia via membrane microdomains and a targeting motif. J. Am. Soc. Nephrol. 20, 2570–2580 (2009).
Follit, J. A., Li, L., Vucica, Y. & Pazour, G. J. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. J. Cell Biol. 188, 21–28 (2010).
Nachury, M. V., Seeley, E. S. & Jin, H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 26, 59–87 (2010).
Iomini, C., Tejada, K., Mo, W., Vaananen, H. & Piperno, G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J. Cell Biol. 164, 811–817 (2004).
Besschetnova, T. Y. et al. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 20, 182–187 (2010).
Pitaval, A., Tseng, Q., Bornens, M. & Théry, M. Cell shape and contractility regulate ciliogenesis in cell cycle-arrested cells. J. Cell Biol. 191, 303–312 (2010). By growing cells on micropatterned substrates, the authors provide evidence that cell shape and spreading influences ciliogenesis.
Parker, J. D. K. et al. Centrioles are freed from cilia by severing prior to mitosis. Cytoskeleton 67, 425–430 (2010).
Pan, J., Wang, Q. & Snell, W. J. An aurora kinase is essential for flagellar disassembly in Chlamydomonas. Dev. Cell 6, 445–451 (2004).
Pan, J. & Snell, W. J. Chlamydomonas shortens its flagella by activating axonemal disassembly, stimulating IFT particle trafficking, and blocking anterograde cargo loading. Dev. Cell 9, 431–438 (2005).
Kinzel, D. et al. Pitchfork regulates primary cilia disassembly and left-right asymmetry. Dev. Cell 19, 66–77 (2010).
Pugacheva, E. N., Jablonski, S. A., Hartman, T. R., Henske, E. P. & Golemis, E. A. HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129, 1351–1363 (2007).
Hubbert, C. et al. HDAC6 is a microtubule-associated deacetylase. Nature 417, 455–458 (2002).
Anderson, C. T. & Stearns, T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Curr. Biol. 19, 1498–1502 (2009).
Thomas, J. et al. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol. Cell 102, 499–513 (2010).
Stolc, V., Samanta, M. P., Tongprasit, W. & Marshall, W. F. Genome-wide transcriptional analysis of flagellar regeneration in Chlamydomonas reinhardtii identifies orthologs of ciliary disease genes. Proc. Natl Acad. Sci. USA 102, 3703–3707 (2005). The first transcriptomic analysis of the genomic programme that is activated during cilia assembly.
Yu, X., Ng, C. P., Habacher, H. & Roy, S. Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nature Genet. 40, 1445–1453 (2008).
Engel, B. D., Ludington, W. B. & Marshall, W. F. Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J. Cell Biol. 187, 81–89 (2009).
Pedersen, L. B. et al. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr. Biol. 15, 262–266 (2005).
Tsao, C. & Gorovsky, M. A. Different effects of Tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Mol. Biol. Cell 19, 1450–1461 (2008).
Pedersen, L. B., Geimer, S. & Rosenbaum, J. L. Dissecting the molecular mechanisms of intraflagellar transport in Chlamydomonas. Curr. Biol. 16, 450–459 (2006).
Engel, B. D. et al. Total internal reflection fluorescence (TIRF) microscopy of Chlamydomonas flagella. Methods Cell Biol. 93, 157–177 (2009).
Sleigh, M. A. The Biology of Cilia and Flagella. (Macmillan New York, 1962).
Hartman, T. R. et al. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum. Mol. Genet. 18, 151–163 (2009).
DiBella, L. M., Park, A. & Sun, Z. Zebrafish Tsc1 reveals functional interactions between the cilium and the TOR pathway. Hum. Mol. Genet. 18, 595–606 (2009).
Bonnet, C. S. et al. Defects in cell polarity underlie TSC and ADPKD-associated cystogenesis. Hum. Mol. Genet. 18, 2166–2176 (2009).
Omori, Y. et al. Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc. Natl Acad. Sci. USA 107, 22671–22676 (2010).
Tammachote, R. et al. Ciliary and centrosomal defects associated with mutation and depletion of the Meckel syndrome genes MKS1 and MKS3. Hum. Mol. Genet. 18, 3311–3323 (2009).
Williams, C. L., Masyukova, S. V. & Yoder, B. K. Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. J. Am. Soc. Nephrol. 21, 782–793 (2010).
Wemmer, K. A. & Marshall, W. F. Flagellar length control in Chlamydomonas — a paradigm for organelle size regulation. Int. Rev. Cytol. 260, 175–212 (2007).
Berman, S. A., Wilson, N. F., Haas, N. A. & Lefebvre, P. A. A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr. Biol. 13, 1145–1149 (2003). Describes the identification of a MAP kinase family member, LF4, that regulates flagellar length via an unknown mechanism.
Tam, L., Wilson, N. F. & Lefebvre, P. A. A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J. Cell Biol. 176, 819–829 (2007).
Ko, H. W. et al. Broad-minded links cell cycle-related kinase to cilia assembly and Hedgehog signal transduction. Dev. Cell 18, 237–247 (2010).
Wiese, M., Kuhn, D. & Grünfelder, C. G. Protein kinase involved in flagellar-length control. Eukaryotic Cell 2, 769–777 (2003).
Wilson, N. F. & Lefebvre, P. A. Regulation of flagellar assembly by glycogen synthase kinase 3 in Chlamydomonas reinhardtii. Eukaryotic Cell 3, 1307–1319 (2004).
Levy, E. M. Flagellar elongation as a moving boundary problem. Bull. Math. Biol. 36, 265–273 (1974).
Child., F. M. In Cell Reproduction: In Honor of Daniel Mazia. ICN-UCLA Symposia on Molecular and Cellular Biology (Eds Dirksen, E. R., Prescott, D. M. & Fox, C. F.) Vol. XII (Academic Press, New York, 1978).
Bressloff, P. C. Stochastic model of intraflagellar transport. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 73, 061916 (2006).
Stephens, R. E. Ciliogenesis in sea urchin embryos−a subroutine in the program of development. Bioessays 17, 331–340 (1995).
Walther, Z., Vashishtha, M. & Hall, J. L. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J. Cell Biol. 126, 175–188 (1994).
Pazour, G. J., Agrin, N., Leszyk, J. & Witman, G. B. Proteomic analysis of a eukaryotic cilium. J. Cell Biol. 170, 103–113 (2005).
Tam, L., Dentler, W. L. & Lefebvre, P. A. Defective flagellar assembly and length regulation in LF3 null mutants in Chlamydomonas. J. Cell Biol. 163, 597–607 (2003).
Afzelius, B. A. Cilia-related diseases. J. Pathol. 204, 470–477 (2004).
Lechtreck, K. F., Delmotte, P., Robinson, M. L., Sanderson, M. J. & Witman, G. B. Mutations in hydin impair ciliary motility in mice. J. Cell Biol. 180, 633–643 (2008).
Acknowledgements
We thank B. Engel and members of the Marshall laboratory for helpful discussions and editorial comments. We also thank S. Nonaka for providing the nodal cilia picture. We apologize to those authors whose work we could not cite owing to space limitations. This work was supported by the US National Institutes of Health and the W. M. Keck Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Leftward flow
-
Flow of extraembryonic fluid across the node surface that moves to the left side of the animal.
- Node
-
The anterior end of the primitive streak. Leftward flow in the node is important to determine the left–right axis of the body.
- Axoneme
-
The insoluble microtubule-based structural scaffold of a cilium.
- Basal body
-
A centriole that is acting to nucleate a cilium.
- B tubule
-
The incomplete second microtubule that, together with the A tubule, forms the outer doublet of the ciliary axoneme.
- Primary cilium
-
A cilium, the basal body of which is the mother centriole that the cell inherited during the previous mitosis. The term is meant to contrast with 'secondary cilia', which refers to any other cilia that form later in the cell cycle.
- Centriole
-
A cylindrical array of nine microtubule triplets that is found in the core of the centrosome.
- Polyglutamylation
-
Modification by addition of multiple glutamate residues onto a protein.
- Bardet–Biedl syndrome
-
A ciliopathy that is characterized by obesity, retinitis pigmentosa, polydactyly and cognitive disability.
- Endocytosis
-
The pathway by which cells take up molecules from the plasma membrane by forming invaginations that close off to become intracellular vesicles.
- Guanine nucleotide exchange factor
-
A protein that stimulates exchange of GDP for GTP on GTP-binding proteins, including G-proteins and GTPases.
- Ciliary waveform
-
A type of ciliary motility that is characterized by large asymmetrical bending motions, as opposed to the flagellar waveform which is characterized by a symmetrical sine-wave-like bending pattern.
- Nephronophthisis
-
A paediatric kidney cyst disease that is characterized by normal-sized kidneys with abnormally dilated ducts.
Rights and permissions
About this article
Cite this article
Ishikawa, H., Marshall, W. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol 12, 222–234 (2011). https://doi.org/10.1038/nrm3085
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3085
This article is cited by
-
TRPP2 is located in the primary cilia of human non-pigmented ciliary epithelial cells
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
KIF2C is a prognostic biomarker associated with immune cell infiltration in breast cancer
BMC Cancer (2023)
-
GPRC5C regulates the composition of cilia in the olfactory system
BMC Biology (2023)
-
Loss of the centrosomal protein ALMS1 alters lipid metabolism and the regulation of extracellular matrix-related processes
Biology Direct (2023)
-
Single-molecule localization microscopy reveals the ultrastructural constitution of distal appendages in expanded mammalian centrioles
Nature Communications (2023)