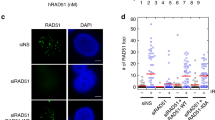
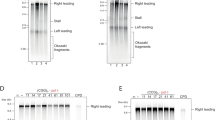
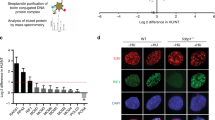
Key Points
-
Replication forks can stall because of an encounter with a DNA-template lesion. The template damage can be from either exogenous or endogenous sources.
-
Stalled forks can lead to genomic instability. To suppress this potential instability, the bacterial cell uses specialized replication-restart pathways that enable fork reactivation outside of the origin of replication.
-
In general, restart involves three steps: processing of the stalled fork to generate proper strand configurations; structure-specific recognition of the stalled fork by one of the restart pathways and assembly of a replisome; and removal of the blocking DNA lesion.
-
Recent findings have shown that the restart systems can prime synthesis of both the nascent leading and lagging strands. This unexpected property indicates that replication can resume downstream of the blocking DNA lesion prior to its removal, presumably leaving a gap behind that would be filled by homologous recombination.
-
Although no obvious homologues of replication-restart components have been identified in eukaryotes, gaps in nascent DNA have been detected in various higher organisms, implying similar and universal fork repair mechanisms between species.
Abstract
Failure to reactivate either stalled or collapsed replication forks is a source of genomic instability in both prokaryotes and eukaryotes. In prokaryotes, dedicated fork repair systems that involve both recombination and replication proteins have been identified genetically and characterized biochemically. Replication conflicts are solved through several pathways, some of which require recombination and some of which operate directly at the stalled fork. Some recent biochemical observations support models of direct fork repair in which the removal of the blocking template lesion is not always required for replication restart.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cox, M. M. et al. The importance of repairing stalled replication forks. Nature 404, 37–41 (2000).
Kreuzer, K. N. Interplay between DNA replication and recombination in prokaryotes. Annu. Rev. Microbiol. 59, 43–67 (2005).
Michel, B., Grompone, G., Flores, M. J. & Bidnenko, V. Multiple pathways process stalled replication forks. Proc. Natl Acad. Sci. USA 101, 12783–12788 (2004).
Sandler, S. J. & Marians, K. J. Role of PriA in replication fork reactivation in Escherichia coli. J. Bacteriol. 182, 9–13 (2000).
Cox, M. M. The nonmutagenic repair of broken replication forks via recombination. Mutat. Res. 510, 107–120 (2002).
Marians, K. J. PriA-directed replication fork restart in Escherichia coli. Trends Biochem. Sci. 25, 185–189 (2000).
McGlynn, P. & Lloyd, R. G. Recombinational repair and restart of damaged replication forks. Nature Rev. Mol. Cell Biol. 3, 859–870 (2002).
Zhang, C., Roberts, T. M., Yang, J., Desai, R. & Brown, G. W. Suppression of genomic instability by SLX5 and SLX8 in Saccharomyces cerevisiae. DNA Repair 5, 336–346 (2005).
Lambert, S., Watson, A., Sheedy, D. M., Martin, B. & Carr, A. M. Gross chromosomal rearrangements and elevated recombination at an inducible site-specific replication fork barrier. Cell 121, 689–702 (2005).
Myung, K., Chen, C. & Kolodner, R. D. Multiple pathways cooperate in the suppression of genome instability in Saccharomyces cerevisiae. Nature 411, 1073–1076 (2001). Elaborates the hypothesis, on the basis of genetic analyses, that gross chromosomal rearrangements, which are the hallmarks of cancer cells, are the result of DNA-replication mishaps. Also, they are normally suppressed in S. cerevisiae cells by multiple pathways that include checkpoint functions, telomere-processing factors and recombination proteins.
McGlynn, P. & Lloyd, R. G. Genome stability and the processing of damaged replication forks by RecG. Trends Genet. 18, 413–419 (2002).
Jones, J. M. & Nakai, H. Duplex opening by primosome protein PriA for replisome assembly on a recombination intermediate. J. Mol. Biol. 289, 503–516 (1999).
Heller, R. C. & Marians, K. J. The disposition of nascent strands at stalled replication forks dictates the pathway of replisome loading during restart. Mol. Cell 17, 733–743 (2005).
Hall, M. C. & Matson, S. W. Helicase motifs: the engine that powers DNA unwinding. Mol. Microbiol. 34, 867–877 (1999).
McGlynn, P., Al-Deib, A. A., Liu, J., Marians, K. J. & Lloyd, R. G. The DNA replication protein PriA and the recombination protein RecG bind D-loops. J. Mol. Biol. 270, 212–221 (1997).
Nurse, P., Liu, J. & Marians, K. J. Two modes of PriA binding to DNA. J. Biol. Chem. 274, 25026–25032 (1999).
Liu, J., Nurse, P. & Marians, K. J. The ordered assembly of the φX174-type primosome. III. PriB facilitates complex formation between PriA and DnaT. J. Biol. Chem. 271, 15656–15661 (1996).
Lopper, M., Holton, J. M. & Keck, J. L. Crystal structure of PriB, a component of the Escherichia coli replication restart primosome. Structure 12, 1967–1975 (2004).
Shioi, S. et al. Crystal structure of a biologically functional form of PriB from Escherichia coli reveals a potential single-stranded DNA-binding site. Biochem. Biophys. Res. Commun. 326, 766–776 (2005).
Liu, J. & Marians, K. J. PriA-directed assembly of a primosome on D loop DNA. J. Biol. Chem. 274, 25033–25041 (1999).
Ng, J. Y. & Marians, K. J. The ordered assembly of the φX174-type primosome. I. Isolation and identification of intermediate protein-DNA complexes. J. Biol. Chem. 271, 15642–15648 (1996).
Liu, J., Xu, L., Sandler, S. J. & Marians, K. J. Replication fork assembly at recombination intermediates is required for bacterial growth. Proc. Natl Acad. Sci. USA 96, 3552–3555 (1999).
Xu, L. & Marians, K. J. PriA mediates DNA replication pathway choice at recombination intermediates. Mol. Cell 11, 817–826 (2003).
Nurse, P., Zavitz, K. H. & Marians, K. J. Inactivation of the Escherichia coli PriA DNA replication protein induces the SOS response. J. Bacteriol. 173, 6686–6693 (1991).
Lee, E. H. & Kornberg, A. Replication deficiencies in priA mutants of Escherichia coli lacking the primosomal replication n' protein. Proc. Natl Acad. Sci. USA 88, 3029–3032 (1991).
Kogoma, T., Cadwell, G. W., Barnard, K. G. & Asai, T. The DNA replication priming protein, PriA, is required for homologous recombination and double-strand break repair. J. Bacteriol. 178, 1258–1264 (1996). This discovery aided in the understanding of the connections between DNA replication, DSB repair and recombination, as well as the importance of PriA-directed primosome assembly for enabling the initiation of DNA replication outside of the origin.
Sandler, S. J., Samra, H. S. & Clark, A. J. Differential suppression of priA2::kan phenotypes in Escherichia coli K-12 by mutations in priA, lexA, and dnaC. Genetics 143, 5–13 (1996).
Mizukoshi, T., Tanaka, T., Arai, K., Kohda, D. & Masai, H. A critical role of the 3′ terminus of nascent DNA chains in recognition of stalled replication forks. J. Biol. Chem. 278, 42234–42239 (2003).
Masai, H., Asai, T., Kubota, Y., Arai, K. & Kogoma, T. Escherichia coli PriA protein is essential for inducible and constitutive stable DNA replication. EMBO J. 13, 5338–5345 (1994).
Sandler, S. J. et al. dnaC mutations suppress defects in DNA replication- and recombination-associated functions in priB and priC double mutants in Escherichia coli K-12. Mol. Microbiol. 34, 91–101 (1999).
Sandler, S. J. Multiple genetic pathways for restarting DNA replication forks in Escherichia coli K-12. Genetics 155, 487–497 (2000). This genetic analysis provided insight into the complex relationship between replication-restart components, outlining a model for multiple restart pathways that contribute to full cellular viability.
McCool, J. D., Ford, C. C. & Sandler, S. J. A dnaT mutant with phenotypes similar to those of a priA2::kan mutant in Escherichia coli K-12. Genetics 167, 569–578 (2004).
Heller, R. C. & Marians, K. J. Unwinding of the nascent lagging strand by Rep and PriA enables the direct restart of stalled replication forks. J. Biol. Chem. 280, 34143–34151 (2005).
Sandler, S. J. Requirements for replication restart proteins during constitutive stable DNA replication in Escherichia coli K-12. Genetics 169, 1799–1806 (2005).
Boonsombat, R., Yeh, S. P., Milne, A. & Sandler, S. J. A novel dnaC mutation that suppresses priB rep mutant phenotypes in Escherichia coli K-12. Mol. Microbiol. 60, 973–983 (2006).
Harinarayanan, R. & Gowrishankar, J. A dnaC mutation in Escherichia coli that affects copy number of ColE1-like plasmids and the PriA–PriB (but not Rep–PriC) pathway of chromosomal replication restart. Genetics 166, 1165–1176 (2004).
Sandler, S. J., McCool, J. D., Do, T. T. & Johansen, R. U. PriA mutations that affect PriA–PriC function during replication restart. Mol. Microbiol. 41, 697–704 (2001).
Jaktaji, R. P. & Lloyd, R. G. PriA supports two distinct pathways for replication restart in UV-irradiated Escherichia coli cells. Mol. Microbiol. 47, 1091–1100 (2003).
Higuchi, K. et al. Fate of DNA replication fork encountering a single DNA lesion during oriC plasmid DNA replication in vitro. Genes Cells 8, 437–449 (2003).
McInerney, P. & O'Donnell, M. Functional uncoupling of twin polymerases: mechanism of polymerase dissociation from a lagging-strand block. J. Biol. Chem. 279, 21543–21551 (2004). A clear account of the fate of the E. coli replisome following an encounter with a defined replication block on the lagging-strand template. It describes the functional uncoupling of the leading-strand and lagging-strand polymerases, even though they remain physically associated.
Svoboda, D. L. & Vos, J. M. Differential replication of a single, UV-induced lesion in the leading or lagging strand by a human cell extract: fork uncoupling or gap formation. Proc. Natl Acad. Sci. USA 92, 11975–11979 (1995).
Thomas, D. C., Veaute, X., Fuchs, R. P. & Kunkel, T. A. Frequency and fidelity of translesion synthesis of site-specific N-2-acetylaminofluorene adducts during DNA replication in a human cell extract. J. Biol. Chem. 270, 21226–21233 (1995).
Thomas, D. C., Veaute, X., Kunkel, T. A. & Fuchs, R. P. Mutagenic replication in human cell extracts of DNA containing site-specific N-2-acetylaminofluorene adducts. Proc. Natl Acad. Sci. USA 91, 7752–7756 (1994).
Cordeiro-Stone, M., Makhov, A. M., Zaritskaya, L. S. & Griffith, J. D. Analysis of DNA replication forks encountering a pyrimidine dimer in the template to the leading strand. J. Mol. Biol. 289, 1207–1218 (1999).
Pages, V. & Fuchs, R. P. Uncoupling of leading- and lagging-strand DNA replication during lesion bypass in vivo. Science 300, 1300–1303 (2003).
Lopes, M., Foiani, M. & Sogo, J. M. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 21, 15–27 (2006).
Goldfless, S. J., Morag, A. S., Belisle, K. A., Sutera, V. A. Jr. & Lovett, S. T. DNA repeat rearrangements mediated by DnaK-dependent replication fork repair. Mol. Cell 21, 595–604 (2006).
Okazaki, R., Okazaki, T., Sakabe, K., Sugimoto, K. & Sugino, A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc. Natl Acad. Sci. USA 59, 598–605 (1968).
Wang, T. C. Discontinuous or semi-discontinuous DNA replication in Escherichia coli? Bioessays 27, 633–636 (2005).
Wang, T. C. & Smith, K. C. Discontinuous DNA replication in a lig-7 strain of Escherichia coli is not the result of mismatch repair, nucleotide-excision repair, or the base-excision repair of DNA uracil. Biochem. Biophys. Res. Commun. 165, 685–688 (1989).
Heller, R. C. & Marians, K. J. Replication fork reactivation downstream of a blocked nascent leading strand. Nature 439, 557–562 (2006). Describes a biochemical reconstitution of replication restart in which a leading-strand block is bypassed through a de novo priming event downstream of the block, thereby generating gaps in the nascent DNA.
Rupp, W. D. & Howard-Flanders, P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J. Mol. Biol. 31, 291–304 (1968). A seminal work in the field of DNA repair that describes the skipping of UV-induced lesions during DNA replication in E. coli , which results in the generation of discontinuities, or gaps, in the nascent DNA. These gaps are subsequently repaired after further incubation.
Iyer, V. N. & Rupp, W. D. Usefulness of benzoylated naphthoylated DEAE-cellulose to distinguish and fractionate double-stranded DNA bearing different extents of single-stranded regions. Biochim. Biophys. Acta 228, 117–126 (1971).
Smith, K. C. DNA synthesis in sensitive and resistant mutants of Escherichia coli B after ultraviolet irradiation. Mutat. Res. 8, 481–495 (1969).
Ganesan, A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J. Mol. Biol. 87, 103–119 (1974).
Mok, M. & Marians, K. J. The Escherichia coli preprimosome and DNA B helicase can form replication forks that move at the same rate. J. Biol. Chem. 262, 16644–16654 (1987).
Berdichevsky, A., Izhar, L. & Livneh, Z. Error-free recombinational repair predominates over mutagenic translesion replication in E. coli. Mol. Cell 10, 917–924 (2002).
Smith, K. C., Wang, T. V. & Sharma, R. C. recA-dependent DNA repair in UV-irradiated Escherichia coli. J. Photochem. Photobiol. B 1, 1–11 (1987).
Tang, M. et al. Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature 404, 1014–1018 (2000).
Kowalczykowski, S. C., Dixon, D. A., Eggleston, A. K., Lauder, S. D. & Rehrauer, W. M. Biochemistry of homologous recombination in Escherichia coli. Microbiol. Rev. 58, 401–465 (1994).
Grompone, G., Sanchez, N., Ehrlich, S. D. & Michel, B. Requirement for RecFOR-mediated recombination in priA mutants. Mol. Microbiol. 52, 551–562 (2004). A genetic analysis of recombination function in cells that lack PriA. It indicates that a prevalence of single-stranded gaps are produced by PriA-independent mechanisms, because repair by the RecFOR proteins becomes essential for viability.
Kuzminov, A. Recombinational repair of DNA damage in Escherichia coli and bacteriophage λ. Microbiol. Mol. Biol. Rev. 63, 751–813 (1999).
McCool, J. D. & Sandler, S. J. Effects of mutations involving cell division, recombination, and chromosome dimer resolution on a priA2::kan mutant. Proc. Natl Acad. Sci. USA 98, 8203–8210 (2001).
Gregg, A. V., McGlynn, P., Jaktaji, R. P. & Lloyd, R. G. Direct rescue of stalled DNA replication forks via the combined action of PriA and RecG helicase activities. Mol. Cell 9, 241–251 (2002).
McGlynn, P. & Lloyd, R. G. Modulation of RNA polymerase by (p)ppGpp reveals a RecG-dependent mechanism for replication fork progression. Cell 101, 35–45 (2000).
Whitby, M. C., Ryder, L. & Lloyd, R. G. Reverse branch migration of Holliday junctions by RecG protein: a new mechanism for resolution of intermediates in recombination and DNA repair. Cell 75, 341–350 (1993).
Cromie, G. A. & Leach, D. R. Control of crossing over. Mol. Cell 6, 815–826 (2000).
Courcelle, J., Crowley, D. J. & Hanawalt, P. C. Recovery of DNA replication in UV-irradiated Escherichia coli requires both excision repair and RecF protein function. J. Bacteriol. 181, 916–922 (1999).
Courcelle, J. & Hanawalt, P. C. RecA-dependent recovery of arrested DNA replication forks. Annu. Rev. Genet. 37, 611–646 (2003).
Rupp, W. D., Wilde, C. E. 3rd, Reno, D. L. & Howard-Flanders, P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J. Mol. Biol. 61, 25–44 (1971).
Bidnenko, V., Ehrlich, S. D. & Michel, B. Replication fork collapse at replication terminator sequences. EMBO J. 21, 3898–3907 (2002).
Donaldson, J. R., Courcelle, C. T. & Courcelle, J. RuvAB and RecG are not essential for the recovery of DNA synthesis following UV-induced DNA damage in Escherichia coli. Genetics 166, 1631–1640 (2004).
Jones, J. M. & Nakai, H. Escherichia coli PriA helicase: fork binding orients the helicase to unwind the lagging strand side of arrested replication forks. J. Mol. Biol. 312, 935–947 (2001).
Cadman, C. J. & McGlynn, P. PriA helicase and SSB interact physically and functionally. Nucleic Acids Res. 32, 6378–6387 (2004).
Tanaka, T. & Masai, H. Stabilization of a stalled replication fork by concerted actions of two helicases. J. Biol. Chem. 281, 3484–3493 (2006).
Al-Deib, A. A., Mahdi, A. A. & Lloyd, R. G. Modulation of recombination and DNA repair by the RecG and PriA helicases of Escherichia coli K-12. J. Bacteriol. 178, 6782–6789 (1996).
Lane, H. E. & Denhardt, D. T. The rep mutation. IV. Slower movement of replication forks in Escherichia coli rep strains. J. Mol. Biol. 97, 99–112 (1975).
Yancey-Wrona, J. E. & Matson, S. W. Bound Lac repressor protein differentially inhibits the unwinding reactions catalyzed by DNA helicases. Nucleic Acids Res. 20, 6713–6721 (1992).
Matson, S. W., Bean, D. W. & George, J. W. DNA helicases: enzymes with essential roles in all aspects of DNA metabolism. Bioessays 16, 13–22 (1994).
Mahdi, A. A., Buckman, C., Harris, L. & Lloyd, R. G. Rep and PriA helicase activities prevent RecA from provoking unnecessary recombination during replication fork repair. Genes Dev. 20, 2135–2147 (2006). Using primarily a synthetic lethality assay that provides a visual display of cell viability, this work brought forth the idea that the helicase activity of Rep and PriA serves to limit the loading of RecA and to limit unnecessary recombination at stalled replication forks.
Flores, M. J., Sanchez, N. & Michel, B. A fork-clearing role for UvrD. Mol. Microbiol. 57, 1664–1675 (2005).
Veaute, X. et al. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. EMBO J. 24, 180–189 (2005).
Lehmann, A. R. Postreplication repair of DNA in ultraviolet-irradiated mammalian cells. J. Mol. Biol. 66, 319–337 (1972).
Meneghini, R. Gaps in DNA synthesized by ultraviolet light-irradiated WI38 human cells. Biochim. Biophys. Acta 425, 419–427 (1976).
Prakash, L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol. Gen. Genet. 184, 471–478 (1981).
Lawrence, C. W. & Christensen, R. B. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J. Bacteriol. 139, 866–876 (1979).
Broomfield, S., Hryciw, T. & Xiao, W. DNA postreplication repair and mutagenesis in Saccharomyces cerevisiae. Mutat. Res. 486, 167–184 (2001).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–233 (2005).
Schekman, R., Weiner, J. H., Weiner, A. & Kornberg, A. Ten proteins required for conversion of φX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J. Biol. Chem. 250, 5859–5865 (1975).
Wickner, S. & Hurwitz, J. Conversion of φX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc. Natl Acad. Sci. USA 71, 4120–4124 (1974).
Kaguni, J. M. & Kornberg, A. Replication initiated at the origin (oriC) of the E. coli chromosome reconstituted with purified enzymes. Cell 38, 183–190 (1984).
Fuller, R. S., Funnell, B. E. & Kornberg, A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell 38, 889–900 (1984).
Baker, T. A., Funnell, B. E. & Kornberg, A. Helicase action of dnaB protein during replication from the Escherichia coli chromosomal origin in vitro. J. Biol. Chem. 262, 6877–6885 (1987).
Baker, T. A., Sekimizu, K., Funnell, B. E. & Kornberg, A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell 45, 53–64 (1986).
Wickner, S. & Hurwitz, J. Interaction of Escherichia coli dnaB and dnaC(D) gene products in vitro. Proc. Natl Acad. Sci. USA 72, 921–925 (1975).
LeBowitz, J. H. & McMacken, R. The Escherichia coli dnaB replication protein is a DNA helicase. J. Biol. Chem. 261, 4738–4748 (1986).
Fang, L., Davey, M. J. & O'Donnell, M. Replisome assembly at oriC, the replication origin of E. coli, reveals an explanation for initiation sites outside an origin. Mol. Cell 4, 541–553 (1999).
Tougu, K., Peng, H. & Marians, K. J. Identification of a domain of Escherichia coli primase required for functional interaction with the DnaB helicase at the replication fork. J. Biol. Chem. 269, 4675–4682 (1994).
Kim, S., Dallmann, H. G., McHenry, C. S. & Marians, K. J. Coupling of a replicative polymerase and helicase: a τ-DnaB interaction mediates rapid replication fork movement. Cell 84, 643–650 (1996).
Yuzhakov, A., Turner, J. & O'Donnell, M. Replisome assembly reveals the basis for asymmetric function in leading and lagging strand replication. Cell 86, 877–886 (1996).
Acknowledgements
We thank S. Keeney, J. Petrini and R. Rothstein for their comments on the manuscript. Studies from the authors' laboratory were supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- DNA-template lesion
-
Any alteration to either the continuity of the DNA strands or the chemical form of the nucleotide bases and phosphodiester backbone.
- Replication fork
-
The branch point between the two template DNA strands where nascent DNA synthesis is ongoing.
- Replisome
-
The protein machine that replicates DNA and that comprises, at a minimum, the replicative DNA polymerase, the replication-fork DNA helicase and the Okazaki-fragment primase.
- Nucleotide-excision repair
-
A template-dependent process by which modified nucleotides are removed from the DNA by the excision of a patch of single-stranded DNA, including nucleotides both upstream and downstream of the affected base, leaving a gap that is subsequently filled in.
- Recombination-dependent replication (RDR)
-
DNA replication that is initiated from a recombinant joint molecule formed by homologous recombination.
- D-loop
-
Displacement loop. Originally referring to a region on mitochondrial DNA where a short RNA displaces one of the template strands. In homologous recombination, it is the displacement of one strand of the duplex by an invading single strand of DNA during a strand-pairing reaction such as that catalysed by RecA.
- Holliday junction
-
The crossover point of exchange of strands between two sister chromosomes during homologous recombination.
- Leading strand
-
The nascent strand of DNA that can be synthesized continuously in the 5′→3′ direction at the replication fork.
- R-loop
-
A displacement loop that contains an RNA strand annealed to one of the template strands.
- Constitutive stable DNA replication
-
A form of recombination-dependent replication that is induced by either rnhA or recG mutations and that is DnaA and RecBCD independent, RecA dependent, chloramphenicol resistant (that is, it does not require protein synthesis) and rifampicin sensitive (that is, it does require transcription).
- Lagging strand
-
The nascent strand of DNA that is synthesized discontinuously in short (1–2 kilobase pair) pieces (Okazaki fragments) at the replication fork.
- Inducible stable DNA replication
-
An SOS-induced form of recombination-dependent replication that is DnaA independent, RecA and RecBCD dependent, and chloramphenicol and rifampicin resistant (that is, neither protein synthesis nor transcription are required).
- Polymerase uncoupling
-
The concept that the replisome can become functionally uncoupled, with the leading-strand and lagging-strand polymerases working independently of one another and of the replication-fork helicase.
- Daughter-strand gap repair
-
Repair of gaps in the nascent DNA using the complementary strands of the sister duplex and catalysed by the RecF-dependent pathway of homologous recombination.
- Branch migration
-
Movement of the Holliday junction in a recombinant joint molecule axially along the length of the DNA molecules. It results in the exchange of strands between, for example, two sister chromosomes.
- Crossover formation
-
Resolution of the Holliday junction in recombinant joint molecules in a manner that results in the exchange of flanking genetic markers.
- SOS response
-
The prokaryotic DNA-damage response.
- Replication-fork collapse
-
The disjunction of the two partially replicated sister duplexes at the replication fork, such as when the replisome encounters a nick in one of the template strands.
- Fork regression
-
Pairing of the nascent strands of DNA at a replication fork. It results in the rewinding of duplex template DNA (that is, the position of the replication fork regresses, moving backwards along the template) and the formation of a Holliday junction.
Rights and permissions
About this article
Cite this article
Heller, R., Marians, K. Replisome assembly and the direct restart of stalled replication forks. Nat Rev Mol Cell Biol 7, 932–943 (2006). https://doi.org/10.1038/nrm2058
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2058
This article is cited by
-
Increasing the efficiency and precision of prime editing with guide RNA pairs
Nature Chemical Biology (2022)
-
Arabidopsis thaliana PrimPol is a primase and lesion bypass DNA polymerase with the biochemical characteristics to cope with DNA damage in the nucleus, mitochondria, and chloroplast
Scientific Reports (2021)
-
The HLTF–PARP1 interaction in the progression and stability of damaged replication forks caused by methyl methanesulfonate
Oncogenesis (2020)
-
Regulation of long non-coding RNAs and genome dynamics by the RNA surveillance machinery
Nature Reviews Molecular Cell Biology (2020)
-
Helicase promotes replication re-initiation from an RNA transcript
Nature Communications (2018)