Abstract
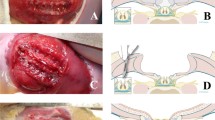
We hypothesize that the neurologic deficit associated with open spina bifida is not directly caused by the primary defect but rather is due to chronic mechanical and chemical trauma since the unprotected neural tissue is exposed to the intrauterine environment. We report here that exposure of the normal spinal cord to the amniotic cavity in midgestational sheep fetuses leads to a human-like open spina bifida with paraplegia at birth, indicating that the exposed neural tissue is progressively destroyed during pregnancy. When open spina bifida was repaired in utero at an intermediate stage, the animals had near-normal neurologic function. The spinal cord was deformed but largely preserved. These findings suggest that secondary neural tissue destruction during pregnancy is primarily responsible for the functional loss and that timely in utero repair of open spina bifida might rescue neurologic function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Shaw, G.M., Jensvold, N.G., Wasserman, C.R. & Lammer, E.J. Epidemiologic characteristics of phenotypically distinct neural tube defects among 0.7 million California births. Teratology 49, 143–149 (1994).
Copp, A.J. Neural tube defects. Trends. Neurosci. 16, 381–383 (1993).
Copp, A.J., Brook, F.A., Estibeiro, J.P., Shum, A.S.W. & Cockroft, D.L. The embryonic development of mammalian neural tube defects. Progr. Neurobiol. 35, 363–403 (1990).
Lemire, R.J. Neural tube defects. J. Am. Med. Assoc. 259, 558–562 (1988).
McLaughlin, J.F. Influence of prognosis on decisions regarding the care of newborns with myelodysplasia. New Engl. J. Med. 312, 1589–1594 (1985).
Campbell, L.R., Dayton, D.H. & Sohal, G.S. A review of human and animal studies on the etiology of neural tube defects. Teratology 34, 171–187 (1986).
Osaka, K., Tanimura, T., Hirayama, A. & Matsumoto, S. Myelomeningocele before birth. J. Neurosurg. 49, 711–724 (1978).
Patten, B.M. Embryological stages in the establishing of myeloschisis with spina bifida. Am. J. Anat. 93, 365–395 (1953).
Keller-Peck, C. & Mullen, R.J. Evidence for late neuronal degeneration in the open neural tube of curly tail mutant mice. Soc. Neurosci. Abst. 19, 181 (1993).
Emery, J.L. & Lendon, R.G. The local cord lesion in neurospinal dysraphism (meningomyelocele). J. Pathol. 110, 83–96 (1973).
Cameron, A.H. The spinal cord lesion in spina bifida cystica. Lancet 2, 171–174 (1956).
Jordan, M.A., Heffez, D.S. & Hutchins, G.M. The relationships of the spinal cord and meninges in meningocele, meningomyelocele and iniencephaly. Teratology 43, 472 (1991).
Schmidt, W. The amniotic fluid compartment: The fetal habitat in Advances in Anatomy Embryology and Cell Biology, (eds Beck, F et al.) 1–100 (Springer, Berlin, New York, 1992).
Lotgering, F.K. & Wallenburg, H.C.S. Mechanisms of production and clearance of amniotic fluid. Semin. Perinatal. 10, 94–102 (1986).
Langer, J.C. Etiology of intestinal damage in gastroschisis. I. Effects of amniotic fluid exposure and bowel constriction in a fetal lamb model. J. Pediatr. Surg. 24, 992–997 (1989).
Tibboel, D. The natural history of gastroschisis during fetal life: Development of the fibrous coating on the bowel loops. Teratology 33, 267–272 (1986).
Niku, S.D., Stein, P.C., Scherz, H.C. & Parsons, C.L. A new method for cytodestruction of bladder epithelium using protamine sulfate and urea. J. Urol. 152, 1025–1028 (1994
Matsuoka, M. & Igisu, H. Comparison of the effects of L-carnitine, D-carnitine and acetyl-L-carnitine on the neurotoxicity of ammonia. Biochem. Pharmacol. 46, 159–164 (1993).
Thévenet, A. & Sengel, P. Naturally occurring wounds and wound healing in chick embryo wings. Roux's Arch. Dev. Biol. 195, 345–354 (1986).
Heffez, D.S., Aryanpur, J., Cuello Rotellini, N.A., Hutchins, G.M. & Freeman, J.M. Intrauterine repair of experimental surgically created dysraphism. Neurosurg. 32, 1005–1010 (1993).
Micheida, M. Intrauterine treatment of spina bifida. Z. Kinderchir. 39, 259–261 (1984).
Adzick, N.S. & Harrison, M.R. Fetal surgical therapy. Lancet 343, 897–902 (1994).
Gilbert, J.N., Jones, K.L., Rorke, L.B., Chernoff, G.F. & James, H.E. Central nervous system anomalies associated with meningomyelocele, hydrocephalus, and the Arnold-Chiari malformation: Reappraisal of theories regarding the pathogenesis of posterior neural tube closure defects. Neurosurgery 18, 559–564 (1986).
Bregman, B. & Goldberger, M.E. Anatomical plasticity and sparing of function after spinal cord damage in neonatal cats. Science 217, 553–555 (1982).
Sypniewski Bregman, B. & Bernstein-Goral, H. Both regenerating and late-developing pathways contribute to transplant-induced anatomical plasticity after spinal cord lesions at birth. Exp. Neural. 112, 49–63 (1991).
Iwashita, Y., Kawaguchi, S. & Murata, M. Restoration of function by replacement of spinal cord segments in the rat. Nature 367, 167–170 (1994).
Korenromp, M.J., Van Gool, J.D., Bruinse, H.W. & Kriek, R. Early fetal leg movements in myelomeningocele. Lancet 1, 917–918 (1986).
Luthy, D.A. Cesarean section before the onset of labor and subsequent motor function in infants with meningomyelocele diagnosed antenatally. New Engl. J. Med. 324, 662–666 (1991).
Harrison, M.R., Jester, J.A. & Ross, N.A. Correction of congenital diaphragmatic hernia in utero. I. The model: Intrathoracic balloon produces fatal pulmonary hypoplasia. Surgery 88, 174–182 (1980).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meuli, M., Meuli-Simmen, C., Hutchins, G. et al. In utero surgery rescues neurological function at birth in sheep with spina bifida. Nat Med 1, 342–347 (1995). https://doi.org/10.1038/nm0495-342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0495-342
This article is cited by
-
Invasive intrauterine Therapien
Der Gynäkologe (2022)
-
Emerging magnetic resonance imaging techniques in open spina bifida in utero
European Radiology Experimental (2021)
-
Open fetal myelomeningocele repair at a university hospital: surgery and pregnancy outcomes
Archives of Gynecology and Obstetrics (2021)