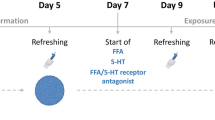
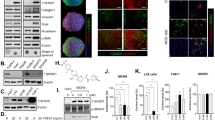
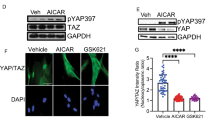
Abstract
Tissue homeostasis requires an effective, limited wound-healing response to injury. In chronic disease, failure to regenerate parenchymal tissue leads to the replacement of lost cellular mass with a fibrotic matrix. The mechanisms that dictate the balance of cell regeneration and fibrogenesis are not well understood1. Here we report that fibrogenic hepatic stellate cells (HSCs) in the liver are negative regulators of hepatocyte regeneration. This negative regulatory function requires stimulation of the 5-hydroxytryptamine 2B receptor (5-HT2B) on HSCs by serotonin, which activates expression of transforming growth factor β1 (TGF-β1), a powerful suppressor of hepatocyte proliferation, through signaling by mitogen-activated protein kinase 1 (ERK) and the transcription factor JunD. Selective antagonism of 5-HT2B enhanced hepatocyte growth in models of acute and chronic liver injury. We also observed similar effects in mice lacking 5-HT2B or JunD or upon selective depletion of HSCs in wild-type mice. Antagonism of 5-HT2B attenuated fibrogenesis and improved liver function in disease models in which fibrosis was pre-established and progressive. Pharmacological targeting of 5-HT2B is clinically safe in humans and may be therapeutic in chronic liver disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gurtner, G.C., Werner, S., Barrandon, Y. & Longaker, M.T. Wound repair and regeneration. Nature 453, 314–321 (2008).
Wallace, K., Burt, A.D. & Wright, M.C. Liver fibrosis. Biochem. J. 411, 1–18 (2008).
Marshall, A. et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 128, 33–42 (2005).
Roskams, T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 25, 3818–3822 (2006).
Malik, R., Selden, C. & Hodgson, H. The role of non-parenchymal cells in liver growth. Semin. Cell Dev. Biol. 13, 425–431 (2002).
Friedman, S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol. Rev. 88, 125–172 (2008).
Passino, M.A., Adams, R.A., Sikorski, S.L. & Akassoglou, K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science 315, 1853–1856 (2007).
Kalinichenko, V.V. et al. Foxf1+/− mice exhibit defective stellate cell activation and abnormal liver regeneration following CCl4 injury. Hepatology 37, 107–117 (2003).
Scobie, B.A. & Summerskill, W.H. Hepatic cirrhosis secondary to obstruction of the biliary system. Am. J. Dig. Dis. 10, 135–146 (1965).
Polimeno, L. et al. Cell proliferation and oncogene expression after bile duct ligation in the rat: evidence of a specific growth effect on bile duct cells. Hepatology 21, 1070–1078 (1995).
Wright, M.C. et al. Gliotoxin stimulates the apoptosis of human and rat hepatic stellate cells and enhances the resolution of liver fibrosis in rats. Gastroenterology 121, 685–698 (2001).
Elrick, L.J. et al. Generation of a monoclonal human single chain antibody fragment to hepatic stellate cells—a potential mechanism for targeting liver anti-fibrotic therapeutics. J. Hepatol. 42, 888–896 (2005).
Douglass, A. et al. Antibody-targeted myofibroblast apoptosis reduces fibrosis during sustained liver injury. J. Hepatol. 49, 88–98 (2008).
Cassiman, D., Libbrecht, L., Desmet, V., Denef, C. & Roskams, T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J. Hepatol. 36, 200–209 (2002).
Omenetti, A. et al. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab. Invest. 87, 499–514 (2007).
Lesurtel, M. et al. Platelet-derived serotonin mediates liver regeneration. Science 312, 104–107 (2006).
Omenetti, A. et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am. J. Gastrointest. Liver Physiol. 300, G303–G315 (2011).
Ruddell, R.G. et al. A role for serotonin (5-HT) in hepatic stellate cell function and liver fibrosis. Am. J. Pathol. 169, 861–876 (2006).
Forbes, I.T., Jones, G.E., Murphy, O.E., Holland, V. & Baxter, G.S. N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea: a novel, high-affinity 5-HT2B receptor antagonist. J. Med. Chem. 38, 855–857 (1995).
Varty, G.B. & Higgins, G.A. Reversal of dizocilpine-induced disruption of prepulse inhibition of an acoustic startle response by the 5-HT2 receptor antagonist ketanserin. Eur. J. Pharmacol. 287, 201–205 (1995).
Fausto, N. Protooncogenes and growth factors associated with normal and abnormal liver growth. Dig. Dis. Sci. 36, 653–658 (1991).
Kim, S.J. et al. Autoinduction of transforming growth factor β 1 is mediated by the AP-1 complex. Mol. Cell. Biol. 10, 1492–1497 (1990).
Smart, D.E. et al. JunD regulates transcription of the tissue inhibitor of metalloproteinases-1 and interleukin-6 genes in activated hepatic stellate cells. J. Biol. Chem. 276, 24414–24421 (2001).
Smart, D.E. et al. JunD is a profibrogenic transcription factor regulated by Jun N-terminal kinase-independent phosphorylation. Hepatology 44, 1432–1440 (2006).
Pillebout, E. et al. JunD protects against chronic kidney disease by regulating paracrine mitogens. J. Clin. Invest. 112, 843–852 (2003).
Yang, L., Besschetnova, T.Y., Brooks, C.R., Shah, J.V. & Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Palmer, M.J. Leads for the treatment of pulmonary hypertension. Expert Opin. Ther. Pat. 19, 575–592 (2009).
Nebigil, C.G. et al. Serotonin 2B receptor is required for heart development. Proc. Natl. Acad. Sci. USA 97, 9508–9513 (2000).
Higgins, G.M. & Anderson, R.M. Experimental pathology of the liver I. Restoration of the liver of white rat following partial surgical removal. Arch. Pathol. 12, 186–202 (1931).
Gieling, R.G. et al. The c-Rel subunit of nuclear factor-κB regulates murine liver inflammation, wound-healing, and hepatocyte proliferation. Hepatology 51, 922–931 (2010).
Arthur, M.J., Friedman, S.L., Roll, F.J. & Bissell, D.M. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J. Clin. Invest. 84, 1076–1085 (1989).
Acknowledgements
This work was funded by grants from the UK Medical Research Council (grant G0700890 to D.A.M., M.C.W. and F.O. and G0900535 to F.O.), the Wellcome Trust (grant WT084961MA to F.O., D.A.M. and M.C.W. and WT086755MA to M.C.W., A.D.B. and D.A.M.). Work in D.A.M.'s lab is also funded by a European Commission FP7 program grant 'INFLA-CARE' (EC Contract No. 223151; http://inflacare.imbb.forth.gr/). M.R.E. was supported by a European Association for Study of the Liver (EASL) Sheila Sherlock fellowship. L.M.'s work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Université Pierre et Marie Curie and by grants from the Fondation de France, the Fondation pour la Recherche Médicale, the French ministry of research (Agence Nationale pour la Recherche) and the European Commission (DEVANX).
Author information
Authors and Affiliations
Contributions
M.R.E. and F.O. performed the majority of the experiments with assistance from L.B.M., J.M., E.E., A.D., M.C.W., F.J., A.M., M.J.P., A.F.L. and L.M. A.D.B. determined the fibrosis scores in the mouse models of liver injury. S.A.W. supplied the human liver tissue for the isolation of hepatocytes and stellate cells. D.A.M. and F.O. conceived of the study, planned the experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
M.C.W. has a financial interest in any commercial use of C1-3 through the licensing agreements of a previous employer but takes no part in and has no influence on any commercialization activities.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods and Supplementary Table 1 (PDF 1808 kb)
Rights and permissions
About this article
Cite this article
Ebrahimkhani, M., Oakley, F., Murphy, L. et al. Stimulating healthy tissue regeneration by targeting the 5-HT2B receptor in chronic liver disease. Nat Med 17, 1668–1673 (2011). https://doi.org/10.1038/nm.2490
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2490
This article is cited by
-
Discovery of a peripheral 5HT2A antagonist as a clinical candidate for metabolic dysfunction-associated steatohepatitis
Nature Communications (2024)
-
Investigating the effects of chronic low-dose radiation exposure in the liver of a hypothermic zebrafish model
Scientific Reports (2023)
-
Cellular crosstalk during liver regeneration: unity in diversity
Cell Communication and Signaling (2022)
-
Protective Effect of Selenium-Enriched Green Tea on Carbon Tetrachloride-Induced Liver Fibrosis
Biological Trace Element Research (2022)
-
Proline-rich tyrosine kinase 2 mediates transforming growth factor-beta-induced hepatic stellate cell activation and liver fibrosis
Scientific Reports (2020)