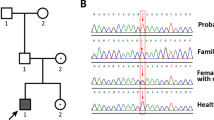
Abstract
Adrenal hypoplasia is a rare, life-threatening congenital disorder. Here we define a new form of syndromic adrenal hypoplasia, which we propose to term MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, and enteropathy) syndrome. By exome sequencing and follow-up studies, we identified 11 patients with adrenal hypoplasia and common extra-adrenal features harboring mutations in SAMD9. Expression of the wild-type SAMD9 protein, a facilitator of endosome fusion, caused mild growth restriction in cultured cells, whereas expression of mutants caused profound growth inhibition. Patient-derived fibroblasts had restricted growth, decreased plasma membrane EGFR expression, increased size of early endosomes, and intracellular accumulation of giant vesicles carrying a late endosome marker. Of interest, two patients developed myelodysplasitc syndrome (MDS) that was accompanied by loss of the chromosome 7 carrying the SAMD9 mutation. Considering the potent growth-restricting activity of the SAMD9 mutants, the loss of chromosome 7 presumably occurred as an adaptation to the growth-restricting condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Clark, A.J., McLoughlin, L. & Grossman, A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet 341, 461–462 (1993).
Metherell, L.A. et al. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat. Genet. 37, 166–170 (2005).
Muscatelli, F. et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature 372, 672–676 (1994).
Meimaridou, E. et al. Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat. Genet. 44, 740–742 (2012).
Prasad, R. et al. Thioredoxin reductase 2 (TXNRD2) mutation associated with familial glucocorticoid deficiency (FGD). J. Clin. Endocrinol. Metab. 99, E1556–E1563 (2014).
Tullio-Pelet, A. et al. Mutant WD-repeat protein in triple-A syndrome. Nat. Genet. 26, 332–335 (2000).
Arboleda, V.A. et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat. Genet. 44, 788–792 (2012).
Hughes, C.R. et al. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 122, 814–820 (2012).
Gineau, L. et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Invest. 122, 821–832 (2012).
Le, S.Q. & Kutteh, W.H. Monosomy 7 syndrome associated with congenital adrenal hypoplasia and male pseudohermaphroditism. Obstet. Gynecol. 87, 854–856 (1996).
McDonald, S. et al. Acquired monosomy 7 myelodysplastic syndrome in a child with clinical features suggestive of dyskeratosis congenita and IMAGe association. Pediatr. Blood Cancer 54, 154–157 (2010).
Topaz, O. et al. A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis. Am. J. Hum. Genet. 79, 759–764 (2006).
Chefetz, I. et al. Normophosphatemic familial tumoral calcinosis is caused by deleterious mutations in SAMD9, encoding a TNF-α responsive protein. J. Invest. Dermatol. 128, 1423–1429 (2008).
Nagamachi, A. et al. Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell 24, 305–317 (2013).
Haase, D. et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood 110, 4385–4395 (2007).
Olney, H.J. & Le Beau, M.M. Evaluation of recurring cytogenetic abnormalities in the treatment of myelodysplastic syndromes. Leuk. Res. 31, 427–434 (2007).
Niemeyer, C.M. & Baumann, I. Myelodysplastic syndrome in children and adolescents. Semin. Hematol. 45, 60–70 (2008).
Duncan, A.W. et al. Aneuploidy as a mechanism for stress-induced liver adaptation. J. Clin. Invest. 122, 3307–3315 (2012).
Amano, N. et al. Radiological evolution in IMAGe association: a case report. Am. J. Med. Genet. A. 146A, 2130–2133 (2008).
Takagi, M. et al. A novel mutation in SOX2 causes hypogonadotropic hypogonadism with mild ocular malformation. Horm. Res. Paediatr. 81, 133–138 (2014).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010).
Aplenc, R., Orudjev, E., Swoyer, J., Manke, B. & Rebbeck, T. Differential bone marrow aspirate DNA yields from commercial extraction kits. Leukemia 16, 1865–1866 (2002).
Tanaka, M., Shimbo, T., Kikuchi, Y., Matsuda, M. & Kaneda, Y. Sterile α motif containing domain 9 is involved in death signaling of malignant glioma treated with inactivated Sendai virus particle (HVJ-E) or type I interferon. Int. J. Cancer 126, 1982–1991 (2010).
Acknowledgements
We thank all the patients and their families for participating in the study; Y. Kaneda (Osaka University) for providing the plasmid; K. Kurosawa and H. Ohashi for establishing skin fibroblast lines; I. Koya for assistance with exome sequencing; and all staff members of the Collaborative Research Resources, Keio University School of Medicine. This study was supported by grants from the Ministry of Health, Labour, and Welfare, Japan (Jitsuyoka Nanbyo-Ippan-014 to T. Hasegawa and Jitsuyoka Nanbyo-Ippan-005 to N. Matsumoto), grants from the Japan Society for the Promotion of Science (23591516 to T.I. and 15K09599 to T. Hasegawa), a grant from the Japan Agency for Medical Research and Development (15ek0109049h0002 to M.F.), a grant from Keio Gijuku Academic Development Funds (to T. Hasegawa), Research Grants for Life Sciences and Medicine from the Keio University Medical Science Fund (to S.N.), and a grant from the Takeda Science Foundation (to S.N.).
Author information
Authors and Affiliations
Contributions
S.N., N.A., T.I., and T. Hasegawa designed the project. N.A., T.I., N.K., and T. Hasegawa recruited patients. M.F., N. Miyake, and N. Matsumoto conducted exome sequencing. S.N., A.S., and J.K. conducted bioinformatics analysis. S.N. and N.A. designed and carried out follow-up genetic analyses and in vitro experiments. S.S. and H.O. conducted electron microscopy. K. Muroya, M.A., K. Toyoshima, K. Miyako, S.K., S.O., K.I., H.I., T.K., T. Hara, M. Kohno, S.Y., H.U., Y.K., K. Tsugawa, A.H., M.M., T.O., Z.K., H.H., M. Kihara, K.S., T.Y., M. Kenmochi, and H.K. clinically characterized the study subjects and collected biological samples. Y.T. and R.F. performed histopathological analysis. S.N. wrote the manuscript with critical input from N.A., T.I., and T. Hasegawa.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Patient enrollment and identified genetic defects.
Forty-one patients (12 syndromic and 29 non-syndromic) with early-onset adrenal insufficiency who had a negative result in newborn screening for congenital adrenal hyperplasia were screened for mutations in known causative genes of primary adrenal insufficiency. As a result, 26 patients were found to have mutations in NR0B1 (n = 12), STAR (n = 10), NNT (n = 2), MC2R (n = 1) or CDKN1C (n = 1). The remaining 15 patients were enrolled in the study. Using whole-exome sequencing and follow-up studies, we identified eight SAMD9 mutation carriers in the original patient cohort.
Supplementary Figure 2 The process of candidate gene discovery.
Using whole-exome sequencing, 15,395 to 20,834 variants were found in six patients. To extract candidate mutations associated with the disease, these variants were filtered by allele frequency and presumed functional impact. In the autosomal recessive model, all variants with an allele frequency of greater than 0.2% in the 1000 Genomes Project database, ESP6500 (Exome Variant Server), ExAC or the Japanese database (HGVD) were excluded. For the dominant model, all variants registered in the above databases or dbSNP138 were excluded. In both models, synonymous variants and non-frameshift indels were excluded. In the recessive model, genes that had one homozygous or two (or more) heterozygous variants were regarded as candidate genes. In the dominant model, genes with at least one heterozygous variant were extracted. In the recessive model, no gene had variants in two or more patients. In the dominant model, one gene (SAMD9), which had variants in four patients, was extracted as a candidate.
Supplementary Figure 3 Conservation of the mutated residues.
Single-letter amino acid Clustal Omega alignments (http://www.clustal.org/omega/) of the residues surrounding the eight missense alterations are shown. The mutated residues are shaded in the species in which each residue is evolutionarily conserved.
Supplementary Figure 4 Partial chromatograms of P3.2 and P10.1, showing mosaic loss of the mutant allele.
In peripheral blood leukocyte samples, obtained before the onset of myelodysplastic syndrome (MDS), the signal derived from the mutated allele was slightly weaker than that derived from the non-mutated allele (red arrowheads), consistent with mosaic monosomy 7. In bone marrow samples, obtained after the onset of MDS, the corresponding signal was mostly derived from the non-mutated allele (red arrows), although the mutated alleles were visible (black arrows). These findings demonstrate the expansion of MDS cells that lost the mutation-carrying chromosome 7. For comparison, wild-type chromatograms and typical heterozygous chromatograms for the identical mutations (P3.1 and P9.1) are shown.
Supplementary Figure 5 Histological findings for two SAMD9-mutation-carrying female patients with adrenal hypoplasia.
The ovaries of P5.1 and P9.1 were hypoplastic and contained reduced numbers of primordial follicles. The thymus was hypoplastic with a decrease in the number of cortical lymphocytes.
Supplementary Figure 6 The inducer-dependent growth-restricting effect of a mutant SAMD9 protein (Arg1293Trp).
Stably transfected HEK293 cells expressing RFP-tagged mutant SAMD9 protein (Arg1293Trp) in the presence of an inducer, cumate, were established. (a) Immunoblot images of cell lysates obtained from the inducible stably transfected HEK293 cells. Addition of the inducer caused dose-dependent expression of the RFP-SAMD9 protein. Note that the degree of overexpression was modest, even with the maximum concentration of the inducer. (b) The effect of the mutant SAMD9 protein (Arg1293Trp) on cell growth was assessed with a time-lapse microscope. The growth-restricting effect of the mutant SAMD9 is shown.
Supplementary Figure 7 Effects of rare polymorphisms in SAMD9 on cell growth.
Rare SNPs were selected if they (i) affected a residue located within ten amino acids of mutated residues (that is, Arg459, Asp769, Asn834, Glu974, Ala1195, Pro1280, Gln1286 or Arg1293), (ii) were predicted to be deleterious by either PolyPhen-2 or SIFT, and (iii) were observed in two or more subjects in the ExAC database (accessed 1 October 2015). Five polymorphisms (E457A, K764E, S838L, R1188Q and V1276D) fulfilled the criteria. These were tested in vitro as MIRAGE-associated mutants. The R1293W substitution was analyzed as a control.
Supplementary Figure 8 Immunoblotting and immunofluorescence studies of SAMD9.
(a) The expression levels of the SAMD9 protein were slightly elevated in P10.1 but were comparable in P1.1 to those in the control. (b) Images of immunofluorescence microscopy. Skin fibroblast cells were transfected with an early endosome marker construct (RFP-Rab5a; red signal) and were incubated with rabbit antibody against SAMD9 and Alexa Fluor 488–conjugated goat anti-rabbit IgG (green signal). No significant difference was observed between the patient-derived cells and control cells.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Figures 1–8 and Supplementary Tables 1 and 2. (PDF 2329 kb)
Rights and permissions
About this article
Cite this article
Narumi, S., Amano, N., Ishii, T. et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet 48, 792–797 (2016). https://doi.org/10.1038/ng.3569
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3569
This article is cited by
-
PARP14 correlates with GBM proliferation and poor prognosis by elevating expression of SAMD/SAMD9L
Irish Journal of Medical Science (1971 -) (2024)
-
Needle in a haystack or elephant in the room? Identifying germline predisposition syndromes in the setting of a new myeloid malignancy diagnosis
Leukemia (2023)
-
Development and function of the fetal adrenal
Reviews in Endocrine and Metabolic Disorders (2023)
-
The International Consensus Classification (ICC) of hematologic neoplasms with germline predisposition, pediatric myelodysplastic syndrome, and juvenile myelomonocytic leukemia
Virchows Archiv (2023)
-
Heterozygotic Brca1 mutation initiates mouse genome instability at embryonic stage
Oncogenesis (2022)