Abstract
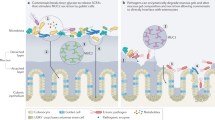
Infectious diarrhea is an important public health problem worldwide. Research has provided new insights into the mechanisms of diarrhea caused by various pathogens that are classified as noninflammatory, inflammatory or invasive. These three groups of organisms cause two diarrheal syndromes—noninflammatory diarrhea and inflammatory diarrhea. The noninflammatory diarrheas are caused by enterotoxin-producing organisms such as Vibrio cholerae and enterotoxigenic Escherichia coli, or by viruses that adhere to the mucosa and disrupt the absorptive and/or secretory processes of the enterocyte without causing acute inflammation or mucosal destruction. Inflammatory diarrhea is caused by two groups of organisms—cytotoxin-producing, noninvasive bacteria (e.g. enteroaggregative Escherichia coli, enterohemorrhagic Escherichia coli and Clostridium difficile), or by invasive organisms (e.g. Salmonella spp., Shigella spp., Campylobacter spp., Entamoeba histolytica). The cytotoxin-producing organisms adhere to the mucosa, activate cytokines and stimulate the intestinal mucosa to release inflammatory mediators. Invasive organisms, which can also produce cytotoxins, invade the intestinal mucosa to induce an acute inflammatory reaction, involving the activation of cytokines and inflammatory mediators. Regardless of the underlying mechanism they use, these various types of pathogen have all successfully evolved to evade and modulate the host defense systems. The mechanisms by which the different pathogens invade the host and cause infectious diarrhea are the topic of this Review.
Key Points
-
Infectious diarrhea is a global health problem and can be classified, based on the pathogenesis and clinical presentation, as noninflammatory diarrhea or inflammatory diarrhea
-
Various host factors protect against enteric infection, and enteropathogens must overcome these to cause disease
-
Common microbial causes of noninflammatory diarrhea are viruses, enterotoxin-producing organisms, Giardia lamblia and Cryptosporidium parvum
-
Enterotoxigenic Escherichia coli and Vibrio cholerae infections cause ADP ribosylation of Gs protein, whereas heat-stable enterotoxin-producing E. coli stimulate cGMP production; both E. coli and V. cholerae increase chloride secretion by crypt cells to produce watery diarrhea
-
Inflammatory diarrhea is caused by two groups of organisms: cytotoxin-producing, noninvasive bacteria (enteroaggregative E. coli, enterohemorrhagic E. coli, and Clostridium difficile); and invasive organisms (e.g. Salmonella spp., Shigella spp., Campylobacter jejuni)
-
Cytotoxin-producing organisms adhere to the mucosa, activate cytokines and stimulate the intestinal mucosa to release inflammatory mediators; invasive organisms, which can also secrete cytotoxins, invade the intestinal mucosa to induce an acute inflammatory reaction and activate cytokines and inflammatory mediators
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kosek M et al. (2003) The global burden of diarrheal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ 81: 197–204
WHO (2005) Cholera, 2004. Wkly Epidemiol Rec 80: 261–268
WHO (2001) Assainissement et diarrhée. Agir contre les infections 2: 1–2
Garthright WE et al. (1988) Estimates of incidence and costs of intestinal infectious diseases in the United States. Public Health Rep 103: 107–115
Steffen R (2005) Epidemiology of traveler's diarrhea. Clin Infect Dis 41: S536–S540
Gaea S and Fasano A (2005) Current concepts in the evaluation, diagnosis and management of acute infectious diarrhea. Curr Opin Pharmacol 5: 559–565
Thielman NM and Guerrant RL (2004) Clinical practice. Acute infectious diarrhea. N Engl J Med 350: 38–47
Giannella RA (2006) Infectious enteritis and proctocolitis and food poisoning. In. Sleisenger & Fordtran's Gastrointestinal and Liver Disease, edn 8 2333–2391 (Ed. Feldman M) Philadelphia: WB Saunders
Giannella RA et al. (1972) Gastric acid barrier to ingested microorganisms in man: In vivo and in vitro studies. Gut 13: 251–256
Musher DM and Musher BL (2004) Contagious acute gastrointestinal infections. N Eng J Med 351: 2417–2428
Rodriguez WJ et al. (1987) Longitudinal study of rotavirus infection and gastroenteritis in families served by a pediatric medical practice: clinical and epidemiologic observations. Pediatr Infect Dis J 6: 170–176
Ciarlet M and Estes MK (2001) Rotavirus and calicivirus infections of the GI tract. Curr Opin Gastroenterol 17: 10–16
WHO (2000) Cholera, 1999. Wkly Epidemiol Rec 75: 249
Morris JG and Black RE (1985) Cholera and other vibrios in the United States. N Engl J Med 312: 343–350
Ramamurthy T et al. (1993) Emergence of novel strain of Vibrio cholerae with epidemic potential in southern and eastern India. Lancet 341: 703–704
Cholera Working Group (1993) Large epidemic of cholera-like disease in Bangladesh caused by Vibrio cholerae 0139 synonym Bengal. Lancet 342: 387–390
Levine MM et al. (1993) Epidemiologic studies of Escherichia coli diarrheal infections in a low socioeconomic level peri-urban community in Santiago, Chile. Am J Epidemiol 138: 849–869
Taylor DN et al. (1988) Clinical and microbiologic features of Shigella and enteroinvasive Escherichia coli infections detected by DNA hybridization. J Clin Microbiol 26: 1362–1366
Slutsker L et al. (1997) Escherichia coli O157:H7 diarrhea in the United States: clinical and epidemiological features. Ann Intern Med 126: 505–513
Nataro J et al. (2006) Diarrheagenic E. coli in Baltimore, Maryland and New Haven, Connecticut. Clin Infect Dis 43: 402–407
Huang DB et al. (2004) Enteroaggregative Escherichia coli: an emerging enteric pathogen. Am J Gastroenterol 99: 383–389
Keusch GT (2002) Shigella and enteroinvasive E. coli In Infections of the GI Tract, edn 2 643–667 (Eds Blaser M. et al.) Philadelphia: Lippincott Williams & Wilkins
Altekruse SF et al. (1999) Campylobacter jejuni: an emerging food borne pathogen. Emerg Infect Dis 5: 28–35
Kyne L et al. (2001) Clostridium difficile. Gastroenterol Clin North Am 3: 753–777
McFarland LV (1995) Epidemiology of infectious and iatrogenic nosocomial diarrhea in a cohort of general medicine patients. Am J Infect Control 23: 295–305
Haque R et al. (2003) Amebiasis. N Engl J Med 348: 1565–1573
Faubert GM et al. (2002) Giardia duodenalis. In Infections of the GI Tract, edn 2 979–1006 (Eds Blaser M. et al.) Philadelphia: Lippincott Williams & Wilkins
Current WL et al. (1983) Human cryptosporidiosis in immunocompetent and immunodeficient persons: studies of an outbreak and experimental transmission. N Engl J Med 308: 1252–1257
Evans CA et al. (1997) Gastric acid secretion and enteric infection in Bangladesh. Trans R Soc Trop Med Hyg 91: 681–685
Hornick RB et al. (1971) The Broad Street pump revisited: response of volunteers to ingested cholera vibrios. Bull NY Acad Med 47: 1181–1191
Gibbons RJ (1982) Review and discussion of role of mucus in mucosal defense. In Recent Advances in Mucosal Immunity, 342–352 (Eds Strober W. et al.) New York: Raven
Dinari G et al. (1986) Effect of guinea pig or monkey colonic mucus on Shigella aggregation and invasion of HeLa cells by Shigella flexneri lb and 2a. Infec Immun 51: 975–978
Justus PG et al. (1982) Myoelectrical effects of C. difficile: motility-altering factor distinct from its cytotoxin and enterotoxin in rabbits. Gastroenterology 83: 836–843
Hentges DJ (1967) Influence of pH on the inhibitory activity of formic and acetic acids fo. Shigella. J Bacteriol 93: 2029–2030
Bohnhoff M and Miller CP (1962) Enhancing susceptibility to Salmonella infection in streptomycin-treated mice. J Infect Dis 111: 117–127
Rabbani GH et al. (1999) Short-chain fatty acids improve clinical, pathologic, and microbiologic features of experimental shigellosis. J Infect Dis 179: 390–397
Elson CO and Mestecky JF (2002) The mucosal immune system. In Infections of the GI Tract, edn 2 127–139 (Eds Blaser M. et al.) Philadelphia: Lippincott Williams & Wilkins
Freter R (1969) Intestinal immunity: studies of the mechanism of action of intestinal immunity in experimental cholera. Tex Rep Biol Med 27: 299
Donnelly MA and Steiner TS (2002) Two non adjacent regions in enteroaggregative Escherichia coli flagellin are required for activation of toll like receptor 5. J Biol Chem 277: 40456–40461
Lehrer RI et al. (1993) Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol 11: 105–128
Wehkamp J et al. (2005) Mechanisms of Disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol 2: 406–415
Hanson LA et al. (1985) Defense of mucosal membranes by antibodies, receptor analogues, and non-specific host factors. Infection 13: S166–S170
Jiang ZD et al. (2003) Genetic susceptibility to enteroaggregative Escherichia coli diarrhea—polymorphism in the interleukin 8 promoter region. J Infect Dis 188: 506–511
Jiang ZD et al. (2006) A common polymorphism in the interleukin 8 gene promoter is associated with Clostridium difficile diarrhea. Am J Gastroenterol. 101: 1112–1116
Mohamed JA et al. (2007) A novel single-nucleotide polymorphism in the lactoferrin gene is associated with susceptibility to diarrhea in North American travelers to Mexico. Clin Infect Dis 44: 945–952
Lindesmith L et al. (2003) Human susceptibility and resistance to Norwalk virus infection. Nat Med 9: 548–553
Kirkpatrick BD et al. (2008) Association between Cryptosporidium infection and human leukocyte antigen class I and class II alleles. J Infect Dis 197: 474–478
Duggal P et al. (2007) The study of associations betwee. Entamoeba histolytica infection and disease with single nucleotide polymorphisms (SNPS) in immune response genes. Presented at the American Society of Tropical Medicine and Hygiene (ASTMH) 56th Annual Meeting: 2007 November 4–8, Philadelphia, PA
Duggal P et al. (2004) Influence of human leukocyte antigen class II alleles on susceptibility to Entamoeba histolytica infection in Bangladeshi children. J Infect Dis 189: 520–526
Williams-Blangero S et al. (2008) Localization of multiple quantitative trait loci influencing susceptibility to infection with Ascaris lumbricoides. J Infect Dis 197: 66–71
Peisong G et al. (2004) An asthma-associated genetic variant of STAT6 predicts low burden of ascaris worm infestation. Genes Immun 5: 58–62
Oria RB et al. (2007) Role of apolipoprotein E4 in protecting children against early childhood diarrhea outcomes and implications for later development. Med Hypotheses 68: 1099–1107
Dhar U et al. (1996) Clinical features, antimicrobial susceptibility and toxin production in Vibrio cholerae O139 infection: comparison with V. cholerae O1 infection. Trans R Soc Trop Med Hyg 90: 402–405
Basu A et al. (2000) Vibrio cholerae 0139 in Calcutta, 1992–1998: incidence, antibiograms, and genotypes. Emerg Infect Dis 6: 139
Sack DA et al. (2004) Cholera. Lancet 363: 223–233
Sánchez J and Holmgren J (2005) Virulence factors, pathogenesis and vaccine protection in cholera and ETEC diarrhea. Curr Opin Immunol 17: 388–398
Shogomori H and Futerman AH (2001) Cholera toxin is found in detergent insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J Biol Chem 276: 9182–9188
Massol RH et al. (2004) Cholera toxin toxicity does not require functional Arf6- and dynamin-dependent endocytic pathways. Mol Biol Cell 15: 3631–3641
Mourad FH et al. (1995) Role of 5-hydroxytryptamine type 3 receptors in rat intestinal fluid and electrolyte secretion induced by cholera and Escherichia coli enterotoxins. Gut 37: 340–345
Evans DG et al. (1975) Plasmid-controlled colonization factor associated with virulence in Escherichia coli enterotoxigenic for humans. Infect Immun 12: 656–667
Moss J and Richardson SH (1978) Activation of adenylate cyclase by heat-labile E. coli enterotoxin. J Clin Invest 62: 281–285
Karlsson KA et al. (1996) Unexpected carbohydrate cross-binding by Escherichia coli heat-labile enterotoxin. Recognition of human and rabbit target cell glycoconjugates in comparison with cholera toxin. Bioorg Med Chem 4: 1919–1928
Wolf MK (1997) Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin Microbiol Rev 10: 569–584
Lundgren O and Svensson L (2001) Pathogenesis of Rotavirus diarrhea. Microbes Infect 3: 1145–1156
Halaihel N et al. (2000) Rotavirus infection impairs intestinal brush-border membrane Na (+) solute co transport activities in young rabbits. Am J Physiol Gastrointest Liver Physiol 279: G587–G596
Langer RC et al. (2001) Characterization of an intestinal epithelial cell receptor recognized by the Cryptosporidium parvum sporozoite ligand CSL. Infect Immun 69: 1661–1670
Simon D et al. (1994) Studies on the pathogenesis of cryptosporidia-incuced diarrhea in HIV-infected individuals. Am J Gastroenterol 89: 2277–2278
Guarino A et al. (1995). Human intestinal cryptosporidiosis: secretory diarrhea and enterotoxic activity in Caco-2 cells. J Infect Dis 171: 976–983
Genta RM et al. (1993) Duodenal morphology and intensity of infection in AIDS-related intestinal cryptosporidiosis. Gastroenterology 105: 1769–1775
Hill DR (1990) Giardia lamblia. In: Principles and Practice of Infectious Diseases 2487–2493 (Eds Mandell GL. et al.) Philadelphia: Churchill Livingstone
Oberhuber G et al. (1997) Giardiasis: a histologic analysis of 567 cases. Scand J Gastroenterol 32: 48–51
Berni Canani R et al. (2006) Inhibitory effect of HIV-1 Tat protein on the sodium-D-glucose symporter of human intestinal epithelial cells. AIDS 20: 5–10
Griffin PM et al. (2002) Escherichia coli O157:H7 and other enterohemorrhagic E. coli. In Infections of the GI Tract, edn 2 627–642 (Eds Blaser M. et al.) Philadelphia: Lippincott Williams & Wilkins
Su C and Brandt LJ (1995) Escherichia coli O157:H7 infection in humans. Ann Intern Med 123: 698–714
Warny M and Kelly CP (2003) Pathogenicity of Clostridium difficile toxins. I. Microbial Pathogenesis and the Intestinal Epithelial Cell, 503 (Ed. Hecht G) Washington, DC: ASM Press
Riegler M et al. (1995) Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest 95: 2004–2011
Wilkins TD and Tucker KD (1989) C. difficile toxin A uses gal-α1–3galβ1–4GlcNAC as a functional receptor. Microecol Ther 19: 225–231
Nataro JP and Kaper JB (1998) Diarrheaegenic E. coli. Clin Microbiol Rev 11: 142–201
Okeke IN et al. (2000) Heterogeneous virulence of enteroaggregative Escherichia coli strains isolated from children in Southwest Nigeria. J Infect Dis 181: 252–260
Sheikh J et al. (2002) A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest 110: 1329–1337
Vial PA et al. (1988) Characterization of enteroadherent-aggregative Escherichia coli, a putative agent of diarrheal disease. J Infect Dis 158: 70–79
Weintraub A (2007) Enteroaggregative E. coli: epidemiology, virulence, and detection. J Med Microbiol 56: 4–8
Phalipon A and Sansonetti PJ (2003) Shigellosis: innate mechanisms of inflammatory destruction of the intestinal epithelium, adaptive immune response, and vaccine development. Crit Rev Immunol 23: 371–401
Fleckenstein JM and Kopecko DJ (2001) Breaching the mucosal barrier by stealth: an emerging pathogenic mechanism for enteroadherent bacterial pathogens. J Clin Invest 107: 27–30
Matkowskyj KA et al. (2000) Galanin-1 receptor up-regulation mediates the excess colonic fluid production caused by infection with enteric pathogens. Nat Med 6: 1048–1051
Grassl GA and Finlay BB (2008) Pathogenesis of enteric Salmonella infections. Curr Opin Gastroenterol 24: 22–26
Oelschlaeger TA et al. (1993) Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA 90: 6884–6888
Hu L and Kopecko DJ (2000) Interactions of Campylobacter with eukaryotic cells: gut luminal colonization and mucosal invasion mechanisms. In Campylobacter, edn 2 191–205 (Eds Nachamkin I and Blaser MJ) Washington, DC: ASM Press
Hickey TE et al. (2000) Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect Immun 68: 6535–6541
Ravdin JI and Guerrant RL (1981) Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica: study with mammalian tissue culture cells and human erythrocytes. J Clin Invest 68: 1305–1313
Moncada D et al. (2003) Entamoeba histolytica cysteine proteinases disrupt the polymeric structure of colonic mucin and alter its protective function. Infect Immun 71: 838–844
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Navaneethan, U., Giannella, R. Mechanisms of infectious diarrhea. Nat Rev Gastroenterol Hepatol 5, 637–647 (2008). https://doi.org/10.1038/ncpgasthep1264
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpgasthep1264
This article is cited by
-
Promising clinical and immunological efficacy of Bacillus clausii spore probiotics for supportive treatment of persistent diarrhea in children
Scientific Reports (2024)
-
Associations between long-term drought and diarrhea among children under five in low- and middle-income countries
Nature Communications (2022)
-
Meteorological and social conditions contribute to infectious diarrhea in China
Scientific Reports (2021)
-
Temperature and risk of infectious diarrhea: a systematic review and meta-analysis
Environmental Science and Pollution Research (2021)
-
Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis
Scientific Reports (2021)