Abstract
Background:
Although chemotherapeutic regimens containing a taxane or platinum agent have been widely recommended for unfavourable carcinoma of unknown primary (CUP), no evidence exists for the superiority of any administered regimens. To date, the efficacy has been mostly assessed in the limited setting of phase II trials, and few attempts have been made to synthesise all available data for survival outcomes.
Methods:
Electronic databases were searched from 1980 to 2011. Survival results were combined for each pre-specified category of regimens using a random-effects model, and meta-regression models were used to adjust for heterogeneity in some known prognostic factors.
Results:
A total of 32 studies were included for meta-analysis. Tendency towards better survival outcome by platinums or taxanes was indicated. After adjustment for important prognostic factors, however, the difference between the platinum-based and non-platinum regimens became no longer significant. Survival benefits by the taxane-based regimens remained significant, with a prolonged median survival time of 1.52 months (P=0.03) and a higher 1-year survival rate of 6.25% (P=0.05), but the benefit did not sustain for 2 years.
Conclusion:
Although no effective therapies have been established, this meta-analysis helps to fill an important gap of evidence. However, caution should still be taken because of the potential unmeasured confounding.
Similar content being viewed by others
Main
The European Society for Medical Oncology guideline lists recommended chemotherapeutic approaches containing platinums and taxanes as commonly used low-toxicity chemotherapy regimens, but stated that no evidence exists for superior efficacy of any of the administered regimens for unfavourable carcinoma of unknown primary (CUP) patients (Fizazi et al, 2011). Although CUP is a relatively common metastatic cancer constituting 3–5% of all human malignancies (McCredie et al, 1991; Levi et al, 2002), the prognosis of CUP is very poor, with a median survival of 3–4 months, and its 5-year survival rate is <10% (Abbruzzese et al, 1994). Certain CUPs, which are defined as favourable prognostic subsets, show a biology and behaviour similar to known metastatic primary carcinoma (Greco and Pavlidis, 2009). However, patients in the favourable subset constitute only a minority of CUP cases and most patients remain unresponsive to any treatment modalities and have a worse prognosis. As no effective therapies have been established through randomised controlled trials (RCTs) for such patients, empirical systemic treatments considering the performance status of patients are widely accepted.
Some efforts for quantitative integration of survival outcomes of previous clinical studies have been made. A descriptive summary estimated a 9-month median survival time for platinum-based chemotherapy by simply taking the median of the included studies’ quoted median survival times (Pentheroudakis et al, 2009). A recent multiple treatment comparison meta-analysis based on a small number of RCTs on chemotherapy regimens in unfavourable CUP patients reported great uncertainty about the survival benefit of any particular regimen (Golfinopoulos et al, 2009). As the efficacy of chemotherapy for patients with unfavourable CUP has mostly been assessed in the setting of small phase II trials without controls, data from limited numbers of RCTs are insufficient evidence for the superiority of any particular regimen in terms of survival prolongation. Another meta-analysis that combined the response rate from 29 phase II trials showed that the chemotherapeutic regimens were not the sole significant parameters and recommended multivariate analysis to consider the heterogeneity of prognostic factors of previous CUP trials (Adenis et al, 2010).
Thus, this study critically evaluated all relevant studies on chemotherapeutic approaches to unfavourable CUPs, which were mostly from single-arm phase II trials, and evaluated whether platinum- or taxane-based regimens could improve survival in patients from the unfavourable subset of CUPs adjusting for some known prognostic factors.
Methods
Search strategy
Ovid MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials were searched comprehensively without language restrictions, with hand searches for further references. The search period was from 1980, when platinum was introduced into the market, to 2011.
We first searched a combination of keywords: (cancer OR carcinoma OR malignancy OR tumour OR neoplasm) AND (unknown primary OR occult primary OR unknown origin) AND (therapy OR therapeutic OR intervention). A team of reviewers in pairs screened the title and abstract of each article. Any discrepancies in the screening were resolved by re-evaluation and discussion. Each full text of the articles selected by the screening was then reviewed to determine its final inclusion.
Inclusion and exclusion criteria
Studies on chemotherapy for the unfavourable subset of CUP were included. Literature on the favourable subsets, such as papillary adenocarcinoma of the peritoneal cavity in women, poorly differentiated carcinoma with a midline distribution, poorly differentiated neuroendocrine carcinomas, adenocarcinoma involving only the axillary lymph nodes in women, squamous cell carcinoma involving cervical lymph nodes, blastic bone metastases and elevated prostate-specific antigen in men, and a single, small, potentially resectable tumour, were excluded. For the diagnosis of CUP, included studies had to evaluate the origin of metastasis at least by abdominal computerised tomography (CT) with either chest CT or plain chest radiography. Phase II and phase III clinical trials were included and consecutive case series performed with the prospective enrolment of participants following clear inclusion criteria were considered for inclusion. Studies evaluating alternating regimens before disease progression or on second-line chemotherapy were excluded. Duplicate publications were excluded by retaining the one with the longest follow-up or the one that reported the data more comprehensively.
Quality assessment
The Methodological Index for Non-Randomised Studies (MINORS) (Slim et al, 2003) was adopted for quality assessment and applied primarily to all the included studies. Assessments for the Risk of Bias were also conducted for the RCTs using the Cochrane guideline (Higgings, 2008).
Data extraction
A pair of reviewers independently extracted relevant data from the studies using a standardised data extraction form. Information on the type of study design, patient follow-up, prognostic factors, chemotherapeutic regimens, and survival outcomes was abstracted. The survival outcomes were extracted using the methods of Tierney et al (2007), and confidence intervals were calculated based on the method of Simon (1986).
Statistical analysis
We first explored a combined estimate of survival outcome for each pre-specified regimen group: platinum vs no-platinum; taxane vs no-taxane; platinum and taxane (P1T1) vs platinum and no-taxane (P1T0) vs no-platinum and taxane (P0T1) vs no-platinum and no-taxane (P0T0). The outcomes considered were the median survival time, and the 1- and 2-year survival rates.
We pooled the results of each outcome for each category by the inverse variance method to generate a crude overall summary with a 95% confidence interval (95% CI) and descriptively contrasted the point estimates between categories. A multiple meta-regression model was then built up for testing the significance of the differences between platinum and no-platinum; taxane and no-taxane; and each of P1T1, P1T0, P0T1, and P0T0 after adjustment for covariates. Being male, a histology of moderate- to well-differentiated adenocarcinoma, poor Eastern Cooperative Oncology Group (ECOG) performance status (⩾2), liver metastasis, and multiple metastatic sites were considered potentially important prognostic factors (Pavlidis and Fizazi, 2005). The year of the study could also influence survival outcomes and was considered a potential source of heterogeneity.
A random-effects model was applied to combine the outcomes. The statistical heterogeneity among the studies was evaluated qualitatively with a forest plot as well as quantitatively with the I2 measure and χ2 test. The funnel plots asymmetry was assessed for small study effects.
Results
Description of the included studies
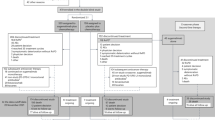
A total of 1389 potentially relevant studies, 1385 from the electronic search and four from the hand searches, were identified, and 1281 were excluded by title/abstract screening. Full texts were retrieved for the remaining 108 studies, and 32 of them met all the criteria for inclusion in the analysis (Figure 1).
There were seven RCTs (Shildt et al, 1983; Dowell et al, 2001; Assersohn et al, 2003; Culine et al, 2003; Palmeri et al, 2006; Huebner et al, 2009; Hainsworth et al, 2010) and one non-randomised trial (Saghatchian et al, 2001) where two treatment arms were evaluated. Six of the included clinical trials primarily explored or estimated the treatment outcome separately; only one phase III trial had a primary objective of directly comparing the two treatment regimens (Hainsworth et al, 2010). Another phase III trial identified by electronic search was excluded because only the abstract was accessible and contacting the author yielded no further information (Gross-Goupil et al, 2009). Both the phase III trials were stopped before enrolment of the planned sample size because of slow accrual.
There was one consecutive case series study (Gill et al, 1991) and the other 23 studies were single arm clinical trials (Raber et al, 1991; Briasoulis et al, 1998, 2000, 2008; Parnis et al, 2000; Greco et al, 2001, 2002; Guardiola et al, 2001; Macdonald et al, 2002; Balana et al, 2003; Mukai et al, 2003, 2010; Park et al, 2004; Piga et al, 2004; Pouessel et al, 2004; El-Rayes et al, 2005; Pittman et al, 2006; Schneider et al, 2007; Pentheroudakis et al, 2008; Hainsworth et al, 2009; Schuette et al, 2009; Yonemori et al, 2009; Moller et al, 2010). Three trials (Briasoulis et al, 1998, 2000; Pentheroudakis et al, 2008) also involved a small proportion of favourable CUP patients in their studies, and we extracted the data only from the unfavourable subset.
In the quality assessment using MINORS, most studies scored ⩾10 points; however, three studies conducted before 2000 scored <10 (Shildt et al, 1983; Raber et al, 1991; Parnis et al, 2000). Two independent reviewers evaluated the responses, which were the primary outcome of the study in only two studies (Culine et al, 2003; Moller et al, 2010). In all, 14 studies reported prospective calculation of the study size (Greco et al, 2002; Macdonald et al, 2002; Pouessel et al, 2004; El-Rayes et al, 2005; Palmeri et al, 2006; Pittman et al, 2006; Schneider et al, 2007; Pentheroudakis et al, 2008; Hainsworth et al, 2009, 2010; Huebner et al, 2009; Schuette et al, 2009; Yonemori et al, 2009; Mukai et al, 2010). Among the RCTs, only one study had a clear description of the method of allocation concealment (Assersohn et al, 2003). No RCT was blinded; however, we did not consider it a major threat to the validity since the outcomes should not be influenced by blinding.
Among the 42 regimens investigated, 35 were evaluated in studies conducted since 2000. In all, 26 regimens were tested based on a sample of 30 to 60 patients. The proportions of male patients in most of the studies were 50–75%. The efficacy of 14 regimens was tested with patients among whom 50–75% had moderate- to well-differentiated adenocarcinoma, and 13 regimens with patients among whom <50% had the histological attribute. The proportion of patients with liver metastasis was <50% in 17 regimens. A total of 24 regimens were assessed with patients among whom <20% had an ECOG performance status ⩾2. The proportion of patients with multiple metastases was 50–75% in 21 regimens. Only two regimens out of 42 were monotherapies; 34 regimens contained platinums and 17 included taxanes. Only one regimen had a follow-up duration shorter than 12 months, and 29 regimens were followed for longer than 24 months. The examined regimens and prognostic profiles are summarised in Table 1.
Survival estimation by regimen groups
The combined results by regimen group, 95% CIs, and statistics for heterogeneity are shown in Figure 2. The overall median survival time was 9.0 months (95% CI: 8.1–9.8), 1-year survival rate 35.6% (95% CI: 32.0–39.3), and 2-year survival rate 18.6% (95% CI: 15.4–21.7) across all chemotherapy regimens.
Combined estimates for the survival by treatment strategy. χ2, chi-squared statistics; n, the number of studies that were included to the regimen groups, I2, I-squared statistics; P1, platinum; P0, no platinum; T1, taxane; T0, no taxane; P1T1, platinum and taxane; P1T0, platinum and no taxane; P0T1, no platinum and taxane; P0T0, no platinum and no taxane; P-value is for the χ2 test.
Most regimens (34 out of 42) contained a platinum component, demonstrating that using platinum in CUP treatment has become common. The majority of platinums were cisplatin or carboplatin, and oxaliplatin was used in only 2 out of the 34 regimens. For the meta-analysis, 26 regimens were included in the platinum group and 6 regimens in the non-platinum group. The platinum-based regimens showed a tendency to have better outcomes in the survival: median survival time of 9.4 vs 7.2 months; 1-year survival rate of 36.9% vs 29.6%; 2-year survival rate of 19.7% vs 11.9% for the platinum regimens vs non-platinum regimens.
When 16 taxane regimens and 16 non-taxane regimens were each combined, a slightly longer median survival (9.6 months) was estimated for the taxane regimens overall than the non-taxane regimens (8.3 months). A greater 1-year survival rate (41.3% vs 30.8%) was observed for the taxane group, and there was no overlapping of 95% CIs between the taxane group and the non-taxane group. However, the tendency towards better results in the taxane group was no longer definite but was sustained for the 2-year survival rate (21.2% vs 16.4%).
Among 14 P1T1, 12 P1T0, 2 P0T1, and 4 P0T0 regimens, we found P1T1 was the best regimen group and P0T0 was the worst for all survival outcomes. While a small number of studies were included, P0T1 tended to be better than P1T0 for median survival time (9.5 vs 8.9 months) and 1-year survival rate (36.6% vs 32.1%).
Investigation of heterogeneity
No significant association was found between the study year and outcomes shown by meta-regression analyses (Table 2). In the univariable meta-regression, the median survival was related to the histology and ECOG performance status. The 1- and 2-year survival probabilities were related to the gender, ECOG performance status, and whether liver metastasis occurred.
Assessment of treatment differences by regimen
The differences we observed in the descriptive comparison of the point estimates of crude overall summaries by platinums became less noticeable and tended to be null after adjustment of the prognostic factors (Table 3). However, the difference in the median survival time by the taxane-containing combination increased to 1.52 months after adjustment for covariates and was statistically significant (P=0.03). The obvious superiority of the 1-year survival rate with taxane-based regimens remained significant (P=0.05), and a possible treatment difference of 6.25% remained. No statistically significant survival benefit was observed from the comparison of the regimens containing platinum, taxane, or both to the chemotherapies with agents other than platinums or taxanes, except for the marginal significance of regimens containing both platinums and taxanes for a median survival time of 2.02 months (P=0.06). The results also suggest a decrease in the 2-year survival rate by taxane regimens without a platinum agent, although it was not statistically significant. The only regimens to have positive results, although not reaching statistical significance, at all end points are the regimens containing both platinum and taxane in comparison to regimens containing neither of them.
Comparison of survival rates from randomised controlled clinical trials
None of the five RCTs providing data for calculating a comparative effect measure showed a hazard ratio (HR) significantly different from one for the overall survival rate (Figure 3). Apart from one out of the five studies, all studies involved at least one regimen containing taxane or platinum. Among the four studies comparing regimens containing taxane with others without taxane, two of the studies showed a direction of effect contrasting with the other two studies. The pooled HR was 0.95 (95% CI: 0.65–1.26), suggesting a nonsignificant difference. In examining the regimens for comparison of platinum against non-platinum, there was also heterogeneity in the direction of treatment effect, which produced a pooled HR of 0.99 (95% CI: 0.67–1.30). For the comparison of regimens containing both taxane and platinum against neither of them, there was a significant heterogeneity between the two included results and they produced a pooled HR of 0.92 (95% CI: 0.44–1.40).
Assessment for funnel plot asymmetry
We observed an asymmetric funnel plot in only the median survival time (P=0.05), but no apparent trend in the 1- or 2-year survival rate. The former indicated an association between the treatment results and study size (Figure 4). We performed the Egger test for the median survival time stratified by taxanes and platinums. The small study effect was more salient in the platinum regimens (P=0.04) than non-platinum regimens (P=0.33) and in non-taxane regimens (P=0.10) than taxane regimens (P=0.96).
Funnel plot for evaluation of the small trial effect for survival. The fitted line corresponds to the regression test for funnel plot asymmetry proposed by Egger et al (1997).
Discussion
An important target of treating cancer patients should be prolonging survival. However, for the unfavourable subset of CUP patients, there has been no clear understanding of the survival benefits by any chemotherapeutic treatment so far, although platinum- or taxane-based regimens have been commonly and empirically used in current clinical practice. The aim of our study was to estimate the survival efficacy of those regimens by systematically evaluating all relevant data in the literature.
In a recently issued guidance by the National Institute for Health and Clinical Excellence on metastasis with unknown primary, a combination of carboplatin and paclitaxel was suggested to have the highest total expected quality-adjusted life years among the therapeutic approaches, but the probability that the combination was cost-effective was <50% at a given willingness to pay threshold in current UK clinical practice (Fowell, 2010; MacReady, 2010). The guidance also indicated a lack of information supporting any particular chemotherapy in terms of survival prolongation in patients with CUP not belonging to a specific favourable group in the currently available literature. Our study suggested that the addition of a taxane to the regimen extended the median survival time by 1.52 months (95% CI: 0.12–2.92) and increased the 1-year survival rate by 6.25% (95% CI: 0.05–12.55). Although the survival benefit did not remain for 2 years, the suggested improvement in the median survival time and the 1-year survival probability, accounting for the heterogeneous settings, can provide clinically meaningful evidence of the survival benefit by including taxane in combinatorial chemotherapy.
The majority of platinums in the included regimens were cisplatin or carboplatin, but oxaliplatin was also used in 2 out of the 34 platinum regimens. Oxaliplatin has a particular indication (Cvitkovic, 1998; Graham and Cassidy, 2012) and may better be considered separately from other platinum agents in clinical use, whereas cisplatin and carboplatin can, for the most part, substitute for each other. For this reason, we conducted a reanalysis by removing the two oxaliplatin regimens from the platinum group, which had little impact on the results but a slight shift towards increasing survival: median survival time from 9.4 months (95% CI: 8.4–10.3) to 9.5 months (95% CI: 8.5–10.5); 1-year survival rate from 36.9% (95% CI: 32.8–41.1) to 38.9% (95% CI: 35.9–42.0); and 2-year survival rate from 19.7% (95% CI: 16.2–23.2) to 20.7% (95% CI: 17.9–23.6). Consequently, the differences between subgroups presented in Table 3 were not influenced much by exclusion of oxaliplatin from the platinum group.
Because RCTs provide another level of evidence, we also analysed them separately by categorising them to assess the effects of containing taxane, platinum, or both. Five RCTs were available with data for calculation of HR. The categorised pooled results suggested slightly favourable results toward taxane, platinum, or both in comparison to regimens without any of them, but failed to confirm the effect, mainly due to heterogeneity between the small number of small-sized studies. A previous meta-analysis of RCTs conducted a multiple treatment comparison, a kind of indirect comparison among different treatments using a Bayesian statistical method, which also failed to demonstrate a survival benefit by any chemotherapeutic regimen for CUP (Golfinopoulos et al, 2009). The analysis also suggested that the hazard ratios favoured platinum (0.69, 95% CI: 0.39–1.28); taxane (0.66, 95% CI: 0.22–2.08); or both (0.81, 95% CI: 0.34–1.89), in comparison with a monotherapy regimen with an agent other than platinum or taxane. Their analysis included a larger number of RCTs because of less restrictive inclusion criteria applied, in terms of method of diagnosis for CUP, than those used in our study. Their results also suggested a great uncertainty with a wider Bayesian version of CIs. Lack of a large enough number of RCTs, especially for phase III, could be a possible explanation of the failure to demonstrate significance, although the possible efficacy of taxane or platinum was suggested consistently. From the five trials included in our meta-analysis of RCTs, only one trial was a study conducted to evaluate a comparative efficacy in the setting of phase III, but the study also stopped early because of slow accrual of patients. The other trials were randomised studies, but still were phase II studies, which aimed to assess efficacy in each treatment arm rather than for investigating a comparative efficacy. In such a circumstance, this study is of importance by filling the lack of certainty in the current evidence by utilising all available information with a maximum control of incomparability.
The funnel plot of the median survival showed an asymmetry indicating a small study effect. The scatter plot of survival outcome against its standard error is supposed to have a symmetric shape of an inverse funnel, representing a wide variability of estimates from small studies and narrow range of those from large studies. Thus, emptiness in the left base of the funnel suggests that small studies have a tendency to report favourable outcomes, which is referred as the small study effect and is often interpreted as an indication of publication bias. However, it is also a common phenomenon in the early development of a new treatment (Lau et al, 2006). The observed small study effect in the current analysis may also be due to such a true heterogeneity (Higgings, 2008), as a short-term benefit from chemotherapy is more likely in patients with high-performance status for tolerating toxicity of the treatment and such patients are more likely to be included in small, early phase clinical trials in the development of new treatment regimens. The stratified funnel plots and Egger test results suggest that the small study effect was more noticeable in the non-taxane or platinum regimens, which may imply that the survival benefits by taxanes were estimated rather conservatively, while those by platinum were still nonsignificant even with possible overestimation.
Our study results should be interpreted with caution because of several limitations. In this setting, a selection of studies with similar populations is particularly difficult, because of the heterogeneous nature of the CUP population, the availability of mostly single institute-based small studies, and the long interval from which the studies were selected. Although we tried to adjust for the differences in known prognostic factors among studies using a meta-regression in the evaluation of the treatment effect, the limitation of this study is that comparisons between those studies can barely substitute for RCTs even after correction for some characteristics across trials. This meta-analysis could still be susceptible to some bias from unmeasured confounders potentially influencing the overall survival, for example, palliative care offered or second-line treatment. Since the included studies have been conducted over a couple of decades, the identification of treatable subsets has changed during the time, which may also cause such heterogeneity as an unmeasurable confounder. Many of the trials, particularly in the first decade, contained some patients who would currently be identified and treated with regimens other than empiric therapy. On the other hand, a significant improvement in the supportive care and the health-care system can also influence the outcomes of the treatments. Most of the included studies were, in fact, found to be carried out within the past decade, and such an evolution effect might therefore have been minimised. We indeed observed lack of a relationship between the survival outcomes and the year of the study (Table 2).
The potential limitations notwithstanding, our study showed that combinations containing a taxane agent demonstrated a prolongation of the median survival time and higher 1-year survival rate in the first-line treatment of unfavourable CUP compared with those without taxanes. However, such a benefit did not seem to be sustained for 2 years. Summaries of available RCTs suggested addition of platinum, taxane, or platinum and taxane to a regimen tends to extend survival of CUP patients; however, those findings based on RCTs were said to be very uncertain owing to a so small number of RCTs. This systematic review and meta-analysis utilising all available clinical trials fills an important gap of certainty in the current evidence by demonstrating a beneficial effect in the use of taxane for unfavourable CUP patients.
Change history
15 January 2013
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P (1994) Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol 12 (6): 1272–1280
Adenis A, Ferte C, Penel N (2010) Phase II trials in patients with carcinoma of unknown primary: a pooled data analysis. Invest New Drugs 28 (2): 178–184
Assersohn L, Norman AR, Cunningham D, Iveson T, Seymour M, Hickish T, Massey A, Prior Y, Hill ME (2003) A randomised study of protracted venous infusion of 5-fluorouracil (5-FU) with or without bolus mitomycin C (MMC) in patients with carcinoma of unknown primary. Eur J Cancer 39 (8): 1121–1128
Balana C, Manzano JL, Moreno I, Cirauqui B, Abad A, Font A, Mate JL, Rosell R (2003) A phase II study of cisplatin, etoposide and gemcitabine in an unfavourable group of patients with carcinoma of unknown primary site. Ann Oncol 14 (9): 1425–1429
Briasoulis E, Fountzilas G, Bamias A, Dimopoulos MA, Xiros N, Aravantinos G, Samantas E, Kalofonos H, Makatsoris T, Mylonakis N, Papakostas P, Skarlos D, Varthalitis I, Pavlidis N (2008) Multicenter phase-II trial of irinotecan plus oxaliplatin [IROX regimen] in patients with poor-prognosis cancer of unknown primary: a hellenic cooperative oncology group study. Cancer Chemother Pharmacol 62 (2): 277–284
Briasoulis E, Kalofonos H, Bafaloukos D, Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C, Kosmidis P, Pavlidis N (2000) Carboplatin plus paclitaxel in unknown primary carcinoma: a phase II Hellenic Cooperative Oncology Group Study. J Clin Oncol 18 (17): 3101–3107
Briasoulis E, Tsavaris N, Fountzilas G, Athanasiadis A, Kosmidis P, Bafaloukos D, Skarlos D, Samantas E, Pavlidis N (1998) Combination regimen with carboplatin, epirubicin and etoposide in metastatic carcinomas of unknown primary site: a Hellenic Co-Operative Oncology Group Phase II Study. Oncology 55 (5): 426–430
Culine S, Lortholary A, Voigt JJ, Bugat R, Theodore C, Priou F, Kaminsky MC, Lesimple T, Pivot X, Coudert B, Douillard JY, Merrouche Y, Allouache J, Goupil A, Negrier S, Viala J, Petrow P, Bouzy J, Laplanche A, Fizazi K (2003) Cisplatin in combination with either gemcitabine or irinotecan in carcinomas of unknown primary site: results of a randomized phase II study-trial for the French Study Group on Carcinomas of Unknown Primary (GEFCAPI 01). J Clin Oncol 21 (18): 3479–3482
Cvitkovic E (1998) Ongoing and unsaid on oxaliplatin: the hope. Br J Cancer 77 (Suppl 4): 8–11
Dowell JE, Garrett AM, Shyr Y, Johnson DH, Hande KR (2001) A randomized phase II trial in patients with carcinoma of an unknown primary site. Cancer 91 (3): 592–597
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109): 629–634
El-Rayes BF, Shields AF, Zalupski M, Heilbrun LK, Jain V, Terry D, Ferris AM, Philip PA (2005) A phase II study of carboplatin and paclitaxel in adenocarcinoma of unknown primary. Am J Clin Oncol 28 (2): 152–156
Fizazi K, Greco FA, Pavlidis N, Pentheroudakis G (2011) Cancers of unknown primary site: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 22 (Suppl 6): vi64–vi68
Fowell A (2010) Metastatic Malignancy of Unknown Primary: Full Guidance. National Collaborating Centre for Cancer: Wales
Gill I, Guaglianone P, Grunberg SM, Scholz M, Muggia FM (1991) High dose intensity of cisplatin and etoposide in adenocarcinoma of unknown primary. Anticancer Res 11 (3): 1231–1235
Golfinopoulos V, Pentheroudakis G, Salanti G, Nearchou AD, Ioannidis JP, Pavlidis N (2009) Comparative survival with diverse chemotherapy regimens for cancer of unknown primary site: multiple-treatments meta-analysis. Cancer Treat Rev 35 (7): 570–573
Graham JS, Cassidy J (2012) Adjuvant therapy in colon cancer. Expert Rev Anticancer Ther 12 (1): 99–109
Greco FA, Burris HA, Litchy S, Barton JH, Bradof JE, Richards P, Scullin DC, Erland JB, Morrissey LH, Hainsworth JD (2002) Gemcitabine, carboplatin, and paclitaxel for patients with carcinoma of unknown primary site: a Minnie Pearl Cancer Research Network study. J Clin Oncol 20 (6): 1651–1656
Greco FA, Gray J, Burris HA, Erland JB, Morrissey LH, Hainsworth JD (2001) Taxane-based chemotherapy for patients with carcinoma of unknown primary site. Cancer J 7 (3): 203–212
Greco FA, Pavlidis N (2009) Treatment for patients with unknown primary carcinoma and unfavorable prognostic factors. Semin Oncol 36 (1): 65–74
Gross-Goupil M, Fourcade A, Blot E, Penel N, Negrier S, Culine S, Merrouche Y, Bouzy J, Laplanche A, Fizazi K (2009) A randomized trial of cisplatin with or without gemcitabine in patients (pts) with carcinoma of an unknown primary (CUP) and without poor prognostic factors: results of the GEFCAPI 02 trial. [Abstract No. 798P] In Annals of Oncology p 248
Guardiola E, Pivot X, Tchicknavorian X, Magne N, Otto J, Thyss A, Schneider M (2001) Combination of cisplatin–doxorubicin–cyclophosphamide in adenocarcinoma of unknown primary site: a phase II trial. Am J Clin Oncol 24 (4): 372–375
Hainsworth JD, Spigel DR, Clark BL, Shipley D, Thompson DS, Farley C, West-Osterfield K, Lane CM, Cescon T, Bury MJ, Greco FA (2010) Paclitaxel/carboplatin/etoposide versus gemcitabine/irinotecan in the first-line treatment of patients with carcinoma of unknown primary site: a randomized, phase III Sarah Cannon Oncology Research Consortium Trial. In CancerJ (Sudbury, MA) 70–75
Hainsworth JD, Spigel DR, Thompson DS, Murphy PB, Lane CM, Waterhouse DM, Naot Y, Greco FA (2009) Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line treatment of patients with carcinoma of unknown primary site. Oncologist 14 (12): 1189–1197
Higgings JGS (2008) Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell: West Sussex
Huebner G, Link H, Kohne CH, Stahl M, Kretzschmar A, Steinbach S, Folprecht G, Bernhard H, Al-Batran SE, Schoffski P, Burkart C, Kullmann F, Otremba B, Menges M, Hoffmann M, Kaiser U, Aldaoud A, Jahn A (2009) Paclitaxel and carboplatin vs gemcitabine and vinorelbine in patients with adeno- or undifferentiated carcinoma of unknown primary: a randomised prospective phase II trial. Br J Cancer 100 (1): 44–49
Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I (2006) The case of the misleading funnel plot. BMJ 333 (7568): 597–600
Levi F, Te VC, Erler G, Randimbison L, La Vecchia C (2002) Epidemiology of unknown primary tumours. Eur J Cancer 38 (13): 1810–1812
Macdonald AG, Nicolson MC, Samuel LM, Hutcheon AW, Ahmed FY (2002) A phase II study of mitomycin C, cisplatin and continuous infusion 5-fluorouracil (MCF) in the treatment of patients with carcinoma of unknown primary site. Br J Cancer 86 (8): 1238–1242
MacReady N (2010) NICE issues guidance on cancer of unknown primary. Lancet Oncol 11 (9): 824
McCredie M, Coates M, Churches T, Taylor R (1991) Cancer incidence in New South Wales, Australia. Eur J Cancer 27 (7): 928–931
Moller AKH, Pedersen KD, Gothelf A, Daugaard G (2010) Paclitaxel, cisplatin and gemcitabine in treatment of carcinomas of unknown primary site, a phase II study. Acta Oncol 49 (4): 423–430
Mukai H, Katsumata N, Ando M, Watanabe T (2010) Safety and efficacy of a combination of docetaxel and cisplatin in patients with unknown primary cancer. Am J Clin Oncol 33 (1): 32–35
Mukai H, Watanabe T, Ando M, Katsumata N (2003) Unknown primary carcinoma: a feasibility assessment of combination chemotherapy with cisplatin and docetaxel. Int J Clin Oncol 8 (1): 23–25
Palmeri S, Lorusso V, Palmeri L, Vaglica M, Porta C, Nortilli R, Ferrau F, Comella G, Massidda B, Danova M (2006) Cisplatin and gemcitabine with either vinorelbine or paclitaxel in the treatment of carcinomas of unknown primary site: results of an Italian multicenter, randomized, phase II study. Cancer 107 (12): 2898–2905
Park YH, Ryoo BY, Choi SJ, Yang SH, Kim HT (2004) A phase II study of paclitaxel plus cisplatin chemotherapy in an unfavourable group of patients with cancer of unknown primary site. Jpn J Clin Oncol 34 (11): 681–685
Parnis FX, Olver IN, Kotasek D, Norman J, Taylor A, Russell J, Patterson K, Keefe D, Marafioti T (2000) Phase II study of epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF) for carcinoma of unknown primary site. Ann Oncol 11 (7): 883–884
Pavlidis N, Fizazi K (2005) Cancer of unknown primary (CUP). Crit Rev Oncol Hematol 54 (3): 243–250
Pentheroudakis G, Briasoulis E, Kalofonos HP, Fountzilas G, Economopoulos T, Samelis G, Koutras A, Karina M, Xiros N, Samantas E, Bamias A, Pavlidis N (2008) Docetaxel and carboplatin combination chemotherapy as outpatient palliative therapy in carcinoma of unknown primary: a multicentre Hellenic Cooperative Oncology Group phase II study. Acta Oncol 47 (6): 1148–1155
Pentheroudakis G, Greco FA, Pavlidis N (2009) Molecular assignment of tissue of origin in cancer of unknown primary may not predict response to therapy or outcome: a systematic literature review. Cancer Treat Rev 35 (3): 221–227
Piga A, Nortilli R, Cetto GL, Cardarelli N, Fedeli SL, Fiorentini G, D’Aprile M, Giorgi F, Parziale AP, Contu A, Montironi R, Gesuita R, Carle F, Cellerino R (2004) Carboplatin, doxorubicin and etoposide in the treatment of tumours of unknown primary site. Br J Cancer 90 (10): 1898–1904
Pittman KB, Olver IN, Koczwara B, Kotasek D, Patterson WK, Keefe DM, Karapetis CS, Parnis FX, Moldovan S, Yeend SJ, Price TJ (2006) Gemcitabine and carboplatin in carcinoma of unknown primary site: a phase 2 Adelaide Cancer Trials and Education Collaborative study. Br J Cancer 95 (10): 1309–1313
Pouessel D, Culine S, Becht C, Ychou M, Romieu G, Fabbro M, Cupissol D, Pinguet F (2004) Gemcitabine and docetaxel as front-line chemotherapy in patients with carcinoma of an unknown primary site. Cancer 100 (6): 1257–1261
Raber MN, Faintuch J, Abbruzzese JL, Sumrall C, Frost P (1991) Continuous infusion 5-fluorouracil, etoposide and cis-diamminedichloroplatinum in patients with metastatic carcinoma of unknown primary origin. Ann Oncol 2 (7): 519–520
Saghatchian M, Fizazi K, Borel C, Ducreux M, Ruffie P, Le Chevalier T, Theodore C (2001) Carcinoma of an unknown primary site: a chemotherapy strategy based on histological differentiation − results of a prospective study. Ann Oncol 12 (4): 535–540
Schneider BJ, El-Rayes B, Muler JH, Philip PA, Kalemkerian GP, Griffith KA, Zalupski MM (2007) Phase II trial of carboplatin, gemcitabine, and capecitabine in patients with carcinoma of unknown primary site. Cancer 110 (4): 770–775
Schuette K, Folprecht G, Kretzschmar A, Link H, Koehne CH, Gruenwald V, Stahl M, Huebner G (2009) Phase II trial of capecitabine and oxaliplatin in patients with adeno- and undifferentiated carcinoma of unknown primary. Onkologie 32 (4): 162–166
Shildt RA, Kennedy PS, Chen TT, Athens JW, O’Bryan RM, Balcerzak SP (1983) Management of patients with metastatic adenocarcinoma of unknown origin: a Southwest Oncology Group study. Cancer Treat Rep 67 (1): 77–79
Simon R (1986) Confidence intervals for reporting results of clinical trials. Ann Intern Med 105 (3): 429–435
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73 (9): 712–716
Tierney JFSL, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. BioMed Central Trials 8: 16
Yonemori K, Ando M, Yunokawa M, Hirata T, Kouno T, Shimizu C, Tamura K, Katsumata N, Hirakawa A, Matsumoto K, Yamanaka Y, Arioka H, Fujiwara Y (2009) Irinotecan plus carboplatin for patients with carcinoma of unknown primary site. Br J Cancer 100 (1): 50–55
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Lee, J., Hahn, S., Kim, DW. et al. Evaluation of survival benefits by platinums and taxanes for an unfavourable subset of carcinoma of unknown primary: a systematic review and meta-analysis. Br J Cancer 108, 39–48 (2013). https://doi.org/10.1038/bjc.2012.516
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.516
Keywords
This article is cited by
-
CUP-Syndrom – die neue ESMO-Leitlinie
best practice onkologie (2023)
-
Clinical outcomes of patients diagnosed with cancer of unknown primary or malignancy of undefined primary origin who were referred to a regional cancer center
International Journal of Clinical Oncology (2023)
-
CUP-Syndrom – die neue ESMO-Leitlinie
Die Radiologie (2023)
-
Das CUP-Syndrom - Stand 2020
InFo Hämatologie + Onkologie (2020)
-
Systemtherapie prognostisch ungünstiger CUP-Syndrome
Der Onkologe (2017)