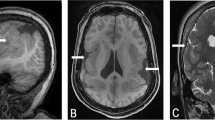
Abstract
Holoprosencephaly (HPE) is a common, severe malformation of the brain that involves separation of the central nervous system into left and right halves. Mild HPE can consist of signs such as a single central incisor, hypotelorism, microcephaly, or other craniofacial findings that can be present with or without associated brain malformations1,2,3. The aetiology of HPE is extremely heterogeneous, with the proposed participation of a minimum of 12 HPE-associated genetic loci as well as the causal involvement of specific teratogens acting at the earliest stages of neurulation4. The HPE2 locus was recently characterized as a 1-Mb interval on human chromosome 2p21 that contained a gene associated with HPE. A minimal critical region was defined by a set of six overlapping deletions and three clustered translocations in HPE patients5. We describe here the isolation and characterization of the human homeobox-containing SIX3 gene from the HPE2 minimal critical region (MCR). We show that at least 2 of the HPE-associated translocation breakpoints in 2p21 are less than 200 kb from the 5´ end of SIX3. Mutational analysis has identified four different mutations in the homeodomain of SIX3 that are predicted to interfere with transcriptional activation and are associated with HPE. We propose that SIX3 is the HPE2 gene, essential for the development of the anterior neural plate and eye in humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cohen, M.M. Perspectives on holoprosencephaly. Part I. Epidemiology, genetics, and syndromology. Teratology 40, 211–235 (1989).
Cohen, M.M. Perspectives on holoprosencephaly. Part III. Spectra, distinctions, continuities, and discontinuities. Am. J. Med. Genet. 34, 371–388 (1989).
Cohen, M.M. & Sulik, K.K. Perspectives on holoprosencephaly. Part II. Central nervous system, craniofacial anatomy, syndrome commentary, diagnostic approach, and experimental studies. J. Craniofac. Genet. Dev. Biol. 12, 196–244 (1992).
Roessler, E. & Muenke, M. Review. Holoprosencephaly: a paradigm for the complex genetics of brain development. J. Inherit. Metab. Dis. 21, 481–497 (1998).
Schell, U. et al. Molecular characterization of breakpoints in patients with holoprosencephaly and definition of the HPE2 critical region 2p21. Hum. Mol. Genet. 5, 223–229 (1996).
Cheyette, B.N.T. et al. The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12, 977–996 (1994).
Serikaku, M.A. & O'Tousa, J.E. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics 138, 1137–1150 (1994).
Burglin, T.R. The evolution of homeobox genes. in Biodiversity and Evolution (eds Arari, R. & Doi, Y.) 291–336 (The National Science Museum Foundation, Tokyo, 1995).
Kawakami, K., Ohno, H., Takizawa, T. & Saito, T. Identification and expression of six family genes in mouse retina. FEBS Lett. 393, 259–263 (1996).
Bovolenta, P., Mallamaci, A. & Boncinelli, E. Cloning and characterization of two chick homeobox genes, members of the Six/sine oculis family, expressed during eye development. Int. J. Dev. Biol. 1 (suppl.), 738–748 (1996).
Zuber, M.E., Perron, M., Bang, A., Jolt, C.D. & Harris, W.A. Molecular cloning and expression analysis of the homeobox gene Six3 in Xenopus laevis. Dev. Biol. 186, 314 (1997).
Loosli, F., Koster, R.W., Carl, M., Crone, A. & Wittbrodt, J. Six3, a medaka homologue of the Drosophila homeobox gene sine oculis is expressed in the anterior embryonic shield and the developing eye. Mech. Dev. 74, 150–164 (1998).
Seo, H.-C., Drivenes, O., Ellingsen, S. & Fjose, A. Expression of two zebrafish homologues of the murine Six3 gene demarcates the initial eye primordia. Mech. Dev. 73, 45–57 (1998).
Toy, J., Yang, J.-M., Leppert, G.S. & Sundin, O.H. The Optix2 homeobox gene is expressed in early precursors of the eye and activates retina-specific genes. Proc. Natl Acad. Sci. USA 96, 10643–10648 (1998).
Kozak, M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc. Natl Acad. Sci. USA 83, 2850–2854 (1986).
Oliver, G. et al. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development 121, 4045–4055 (1995).
Fortin, J.S., Underhill, D.A. & Gros, P. Reciprocal effect of Waardenburg syndrome mutations on DNA binding by the Pax-3 paired domain and homeodomain. Hum. Mol. Genet. 6, 1781–1790 (1997).
Xu, P.-T., Cheng, J., Epstein, J.A. & Maas, R.L. Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc. Natl Acad. Sci. USA 94, 11974–11979 (1997).
Lichter, P. et al. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science 247, 64–69 (1990).
Bucan, M. et al. Physical maps of 4p16.3, the area expected to contain the Huntington disease mutation. Genomics 6, 1–15 (1990).
Acknowledgements
We thank the patients and their families for participation; M. Bucan for input to this work; and D. Telem and J. Morrison for technical assistance. This work was supported by NIH grants HD28732 and HD29862 and by the Division of Intramural Research, National Human Genome Research Institute, NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wallis, D., Roessler, E., Hehr, U. et al. Mutations in the homeodomain of the human SIX3 gene cause holoprosencephaly. Nat Genet 22, 196–198 (1999). https://doi.org/10.1038/9718
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/9718