Abstract
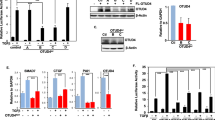
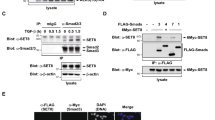
The receptor-regulated Smad proteins are essential intracellular mediators of signal transduction by the transforming growth factor-β (TGF-β) superfamily of growth factors and are also important as regulators of gene transcription. Here we describe a new role for TGF-β-regulated Smad2 and Smad3 as components of a ubiquitin ligase complex. We show that in the presence of TGF-β signalling, Smad2 interacts through its proline-rich PPXY motif with the tryptophan-rich WW domains of Smurf2, a recently identified E3 ubiquitin ligases. TGF-β also induces the association of Smurf2 with the transcriptional co-repressor SnoN and we show that Smad2 can function to mediate this interaction. This allows Smurf2 HECT domain to target SnoN for ubiquitin-mediated degradation by the proteasome. Thus, stimulation by TGF-β can induce the assembly of a Smad2–Smurf2 ubiquitin ligase complex that functions to target substrates for degradation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Attisano, L. & Wrana, J. L. Signal transduction by members of the transforming growth factor-β superfamily. Cyto. Growth Factor Rev. 7, 327–339 (1996).
Heldin, C.-H., Miyazono, K. & ten Dijke, P. TGF-β signalling from cell membrane to nucleus through SMAD proteins. Nature 390, 465–471 (1997).
Massagué, J. TGF-β signal transduction. Annu. Rev. Biochem. 67, 753–91 (1998).
Derynck, R., Zhang, Y. & Feng, X.-H. Smads: transcriptional activators of TGF-β responses. Cell 95, 737–740 (1998).
Massagué, J. & Chen, Y. G. Controlling TGF-β signalling. Genes Dev. 14, 627–644 (2000).
Miyazono, K. TGF-β signalling by Smad proteins. Cyto. Growth Factor Rev. 11, 15–22 (2000).
Wrana, J. L. Regulation of Smad activity. Cell 100, 189–192 (2000).
Macías-Silva, M. et al. MADR2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signalling. Cell 87, 1215–1224 (1996).
Kretzschmar, M., Liu, F., Hata, A., Doody, J. & Massagué, J. The TGF-β family mediator Smad1 is phosphorylated directly and activated functionally by the BMP receptor kinase. Genes Dev. 11, 984–995 (1997).
Abdollah, S. et al. TβRI phosphorylation of Smad2 on Ser 465 and 467 is required for Smad2/Smad4 complex formation and signalling. J. Biol. Chem. 272, 27678–27685 (1997).
Souchelnytskyi, S. et al. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for Transforming Growth Factor-β signalling. J. Biol. Chem. 272, 28107–28115 (1997).
Attisano, L. & Wrana, J. L. Smads as transcriptional co-modulators. Curr. Opin. Cell Biol. 12, 235–243 (2000).
Wotton, D., Lo, R. S., Lee, S. & Massagué, J. A Smad transcriptional co-repressor. Cell 97, 29–39 (1999).
Luo, K. et al. The Ski oncoprotein interacts with the Smad proteins to repress TGF-β signalling. Genes Dev. 13, 2196–2206 (1999).
Akiyoshi, S. et al. c-Ski acts as a transcriptional co-repressor in transforming growth factor-β signalling through interaction with Smads. J. Biol. Chem. 274, 35269–35277 (1999).
Stroschein, S. L., Wang, W., Zhou, S., Zhou, Q. & Luo, K. Negative feedback regulation of TGF-β signalling by the SnoN oncoprotein. Science 286, 771–774 (1999).
Sun, Y. et al. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signalling. Mol. Cell 4, 499–509 (1999).
Sun, Y., Liu, X., Ng-Eaton, E., Lodish, H. F. & Weinberg, R. A. SnoN and Ski proto-oncogene proteins are rapidly degraded in response to transforming growth factor-β signalling. Proc. Natl Acad. Sci.USA 96, 12442–12447 (1999).
Xu, W. et al. Ski acts as a co-repressor with Smad2 and Smad3 to regulate the response to type β transforming growth factor. Proc. Natl Acad. Sci.USA 97, 5924–5929 (2000).
Hayashi, H. et al. The MAD-related protein Smad7 associates with the TGF-β receptor and functions as an antagonist of TGF-β signalling. Cell 89, 1165–1173 (1997).
Nakao, A. et al. Identification of Smad7, a TGF-β-inducible antagonist of TGF-β signalling. Nature 389, 631–635 (1997).
Imamura, T. et al. Smad6 inhibits signalling by the TGF-β superfamily. Nature 389, 622–626 (1997).
Bonifacino, J. S. & Weissman, A. M. Ubiquitin and the control of protein fate in the secretory and endocytic pathways. Annu. Rev. Cell Biol. 14, 19–57 (1998).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Deshaies, R. J. SCF and cullin/ring H2-based ubiquitin ligases. Annu. Rev. Cell Dev. Biol. 15, 435–467 (1999).
Jackson, P. K. et al. The lore of the RINGs: substrate recognition and catalysis by ubiquitin ligases. Trends Cell Biol. 10, 429–439 (2000).
Rotin, D. in Current Topics in Microbiology and Immunology (ed. Pawson, A. S.) 115–133 (Springer-Verlag, Berlin, 1997).
Kay, B. K., Williamson, M. P. & Sudol, M. The importance of being proline: the interaction of proline-rich motifs in signalling proteins with their cognate domains. FASEB J. 14, 231–241 (2000).
Zhu, H., Kavsak, P., Abdollah, S., Wrana, J. L. & Thomsen, G. H. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature 400, 687–693 (1999).
Lin, X., Liang, M. & Feng, X.-H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in transforming Growth Factor-β signalling. J. Biol. Chem. 275, 36818–36822 (2000).
Zhang, Y., Chang, C., Gehling, D. J., Hemmati-Brivanlou, A. & Derynck, R. Regulation of Smad degradation and activity by Smurf2, an E3 ubiquitin ligase. Proc. Natl Acad. Sci. USA 98, 974–979 (2001).
Kavsak, P. et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets TGF-β receptor for degradation. Mol. Cell 6, 1365–1375 (2000).
Nomura, T. et al. Ski is a component of the histone deacetylase complex required for transcriptional repression by Mad and thyroid hormone receptor. Genes Dev. 13, 412–423 (1999).
Lo, R. S. & Massagué, J. Ubiquitin-dependent degradation of TGF-β-activated Smad2. Nature Cell Biol. 1, 472–478 (1999).
Yaron, A. et al. Identification of the receptor component of the IκBα ubiquitin ligase. Nature 396, 590–594 (1998).
Winston, J. T. et al. The SCFβ-TRCP–ubiquitin ligase complex associates specifically with phophorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev. 13, 270–283 (1999).
Spencer, E., Jiang, J. & Chen, Z. J. Signal-induced ubiquitination of IκBα by the F-box protein Slimb/β-TrCP. Genes Dev. 13, 284–294 (1999).
Latres, E., Chiaur, D. S. & Pagano, M. The human F-box protein β-TrCP associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18, 849–854 (1999).
Hart, M. et al. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr. Biol. 9, 207–210 (1999).
Acknowledgements
We thank D. Bohmann for HA-ubiquitin, G. Stark for U4A/Jak1 cells and L. Attisano, C. LeRoy and Y. Wang for helpful discussions. This work was supported by grants to J.L.W. from the Canadian Institutes of Health Research and the National Cancer Institute of Canada with funds from the Terry Fox Run. C.G.C. is a Research Fellow of the National Cancer Institute of Canada with funds from the Terry Fox run, and J.L.W. is a Canadian Institutes of Health Research Investigator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bonni, S., Wang, HR., Causing, C. et al. TGF-β induces assembly of a Smad2–Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol 3, 587–595 (2001). https://doi.org/10.1038/35078562
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/35078562
This article is cited by
-
SIRT2 alleviated renal fibrosis by deacetylating SMAD2 and SMAD3 in renal tubular epithelial cells
Cell Death & Disease (2023)
-
Sumoylated SnoN interacts with HDAC1 and p300/CBP to regulate EMT-associated phenotypes in mammary organoids
Cell Death & Disease (2023)
-
The TFEB-TGIF1 axis regulates EMT in mouse epicardial cells
Nature Communications (2022)
-
PIAS1 and TIF1γ collaborate to promote SnoN SUMOylation and suppression of epithelial–mesenchymal transition
Cell Death & Differentiation (2021)
-
A novel negative regulatory mechanism of Smurf2 in BMP/Smad signaling in bone
Bone Research (2020)