Abstract
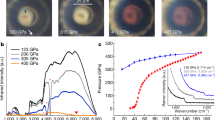
Solid hydrogen, an electrical insulator, is predicted to become an alkali metal under extreme compression, although controversy surrounds the pressure required to achieve this1,2,3. The electrical conductivity of hydrogen as a function of pressure and temperature is of both fundamental and practical interest—metallic hydrogen may be of relevance to planetary interiors4, and has been suggested as a potential high-temperature superconductor5. Calculations1,2 suggest that depairing (destruction of the molecular bond) should occur around 340 GPa, accompanied by the formation of an alkali metal at this pressure1, or at substantially higher pressures2,3. Here we report that solid hydrogen does not become an alkali metal at pressures of up to 342 ± 10 GPa, achieved using a diamond anvil cell. This pressure (which is almost comparable to that at the centre of the Earth) significantly exceeds those reached in earlier experiments—216 GPa (ref. 6) and 191 GPa (ref. 7)—at which hydrogen was found to be non-metallic. The failure of solid hydrogen to become an alkali metal at the extreme pressures reported here has implications for our current theoretical understanding of the solid-state phase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ashcroft, N. W. Pairing instabilities in dense hydrogen. Phys. Rev. B 41, 10963–10971 (1990).
Natoli, V., Martin, R. M. & Ceperley, D. M. Crystal structure of atomic hydrogen. Phys. Rev. Lett. 70, 1952–1955 (1993).
Loubeyre, P. et al. X-ray diffraction and equation of state of hydrogen at megabar pressures. Nature 383, 702–704 (1996).
Saumon, D., Chabrier, G., Hubbard, W. B. & Lunine, J. I. in Strongly Coupled Plasma Physics(eds Ichimaru, S. & Van Horn, H. M.) 111–120 (Univ. Rochester Press, (1993).
Ashcroft, N. W. Metallic hydrogen: a high temperature superconductor. Phys. Rev. Lett. 21, 1748–1749 (1968).
Hemley, R. J., Mao, H.-K., Goncharov, A. F., Hanfland, M. & Struzkin, V. Synchrotron infrared spectroscopy to 0.15 eV of H2 and D2 at megabar pressure. Phys. Rev. Lett. 76, 1667–1670 (1996).
Chen, N. H., Sterer, E. & Silvera, I. F. Extended infrared studies of high pressure hydrogen. Phys. Rev. Lett. 76, 1663–1666 (1996).
Weir, S. T., Vohra, Y. K. & Ruoff, A. L. Pressure induced metallization of BaSe. Phys. Rev. B 35, 874–876 (1987).
Reichlin, R. et al. Evidence for the insulator-metal transition in xenon from optical, x-ray and band structure studies to 170 GPa. Phys. Rev. Lett. 62, 669–672 (1989).
Goettel, K. A., Eggert, J. H. & Silvera, I. F. Optical evidence for the metallization of xenon at 132 (5) GPa. Phys. Rev. Lett. 62, 665–672 (1989).
Luo, H., Desgreniers, S., Vohra, Y. K. & Ruoff, A. L. High pressure optical studies on sulfur to 121 GPa: optical evidence for metallization. Phys. Rev. Lett. 67, 2998–3001 (1991).
Desgreniers, S., Vohra, Y. K. & Ruoff, A. L. Optical response of very high density solid oxygen to 132 GPa. J. Phys. Chem. 94, 1117–1122 (1990).
Jayaraman, A. Diamond anvil cell and high pressure physical investigations. Rev. Mod. Phys. 55, 65–108 (1983).
Kawai, N., Togaya, M. & Mishima, O. Astudy of the metallic hydrogen. Proc. Japan Acad. 51, 630–633 (1975).
Vereshchagin, L. F., Yakovlev, E. N. & Timofeev, YuA. Possibility of transition of hydrogen into the metallic state. JETP Lett. 21, 85–86 (1975).
Hawke, P. S. et al. Observation of electrical conductivity of isotropically compressed hydrogen at megabar pressures. Phys. Rev. Lett. 4, 994–997 (1978).
Burgess, T. G. & Hawke, R. S. Metallic Hydrogen Research 1–26 (Rep. UCID-17977, Lawrence Livermore Lab., CA, (1978).
Mao, H. K. & Hemley, R. J. Optical studies of hydrogen above 200 gigapascals: evidence for metallization by band overlap. Science 244, 1462–1465 (1989).
Mao, H. K., Hemley, R. J. & Hanfland, N. Infrared reflectance measurements of the insulator-metal transition in solid hydrogen. Phys. Rev. Lett. 65, 484–487 (1990).
Hanfland, M., Hemley, R. J. & Mao, H. K. Optical absorption measurements of hydrogen at megabar pressures. Phys. Rev. B 43, 8767–8770 (1991).
Eggert, J. H., Goettel, K. A. & Silvera, I. F. High-pressure dielectric catastrophe and the possibility that the hydrogen A-phase is metallic. Europhys. Lett. 11, 775–781 (1980).
Ashcroft, N. W. Dense hydrogen: the reluctant alkali. Phys. World 8(7), 43–47 (1995).
Weir, S. T., Mitchell, A. C. & Nellis, W. J. Metallization and electrical conductivity of hydrogen in Jupiter. Phys. Rev. Lett. 76, 1860–1863 (1996).
Ruoff, A. L., Xia, H., Luo, H. & Vohra, Y. K. Miniaturization techniques for obtaining static pressures comparable to the pressure at the center of the earth: x-ray diffraction at 416 GPa. Rev. Sci. Instrum. 61, 3830–3833 (1990).
Ruoff, A. L., Xia, H. & Xia, Q. The effect of a tapered aperture on x-ray diffraction from a sample with a pressure gradient: studies on three samples with a maximum pressure of 560 GPa. Rev. Sci. Instrum. 63, 4342–4348 (1992).
Ruoff, A. L. in High Pressure Science and Technology (Proc. AIRAPT XV and EHRPG 33 Conf.)(ed. Trzeciakowski, W.) 511 (World Scientific, Warsaw, (1996).
Birch, J. F. Finite strain isotherm and velocities for single crystal and polycrystalline NaCl at high pressures and 300 °K. J. Geophys. Res. 83, 1257–1269 (1978).
McQueen, R. G., Marsh, S. P., Taylor, J. W., Fritz, J. N. & Carter, W. J. in High Velocity Impact Phenomena(ed. Kinslow, R.) 293–417 (Academic, New York, (1970).
Carter, W. . J., Marsh, S. P., Fritz, J. N. & McQueen, R. J. Accurate Characterization of the High-Pressure Environment(ed. Lloyd, E. C.) 147–158 (Spec. Publ. 326, Natl. Bureau of Standards, Washington DC, (1971).
Hixson, R. S., Boneness, D. A. & Shaner, J. W. Acoustic velocities and phase transitions in molybdenum under strong shock compression. Phys. Rev. Lett. 62, 637–640 (1989).
Christensen, N. E., Ruoff, A. L. & Rodriguez, C. O. Pressure strengthening: a way to multimegabar pressures. Phys. Rev. B 52, 9121–9124 (1993).
Ruoff, A. L., Luo, H., Xia, H. & Vohra, Y. K. The effect of nonhydrostaticity on measuring the pressures in metals by energy dispersive x-ray diffraction. High Press. Res. 6, 183–186 (1991).
Ruoff, A. L. Penultimate static pressure containment consideration and possible applications to metallic hydrogen preparation. Adv. Cryogen. Eng. 18, 435–440 (1973).
Hemley, R. J. et al. X-ray imaging of stress and strain of diamond, iron, and tungsten at megabar pressures. Science 276, 1242–1245 (1997).
Ruoff, A. L., Luo, H. & Vohra, Y. K. The closing diamond anvil optical window in multimegabar research. J. Appl. Phys. 69, 6413–6416 (1991).
Kelly, A. Strong Solids 26 (Oxford Univ. Press, New York, (1996).
Stevenson, D. J. & Ashcroft, N. W. Conduction in fully ionized liquid metals. Phys. Rev. A 9, 782–789 (1974).
Acknowledgements
We acknowledge discussions with N. W. Ashcroft, B. Baranowski and R. M. Martin and thank the CHESS staff for their help. We also acknowledge the role played by B. W. Batterman in creating CHESS. This work was supported by the US NSF and, several years ago in the early stages of this project, by the Cornell Materials Science Center via support by the US NSF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Narayana, C., Luo, H., Orloff, J. et al. Solid hydrogen at 342 GPa: no evidence for an alkali metal. Nature 393, 46–49 (1998). https://doi.org/10.1038/29949
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/29949
This article is cited by
-
Materials under high pressure: a chemical perspective
Applied Physics A (2022)
-
Thermodynamic Properties of the Superconducting State in Metallic Hydrogen: Electronic Correlations, Non-conventional Electron-Phonon Couplings and the Anharmonic Effects
Journal of Superconductivity and Novel Magnetism (2021)
-
A comparative study using state-of-the-art electronic structure theories on solid hydrogen phases under high pressures
npj Computational Materials (2019)
-
Single crystal toroidal diamond anvils for high pressure experiments beyond 5 megabar
Nature Communications (2018)
-
Pressure-induced superconductivity in H2-containing hydride PbH4(H2)2
Scientific Reports (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.