Abstract
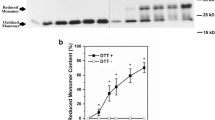
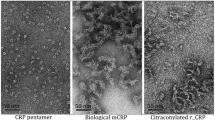
C-Reactive protein (CRP) is composed of five identical noncovalently linked monomers and characterized as an important acute-phase protein. The CRP subunit obtained by denaturing treatments, which is termed modified CRP, has also been widely studied. In the current work, we found that there exists some degree of natural dissociation of CRP in stock solution. This dissociation is critically dependent on the absence of Ca2+. Low pH could enhance the dissociation of CRP, while ionic strength has little effect. Anilinonaphthalenesulfonate (ANS) fluorescence detections indicate that the exposure of hydrophobic surface increases during the dissociation. Acidic pH conditions also induce an increase in ANS fluorescence. This suggests that hydrophobic interactions between CRP subunits may contribute to the study of its pentameric structure. Surface plasmon resonance experiments indicate that monomeric CRP does not specifically bind to phosphatidylcholine-containing membrane as native CRP does. Electron microscopy shows that monomeric CRP binds to negatively charged lipid through electrostatic forces, and such lipid may induce the dissociation of CRP due to the acidic pH in the diffuse double layer near the membrane.
Similar content being viewed by others
REFERENCES
Tillet, W. S., and Francis, T. (1930) J. Exp. Med., 52, 561-571.
Steel, D. M., and Whitehead, A. S. (1994) Immunol. Today, 15, 81-88.
Osmand, A. P., Friedenson, B., Gewurz, H., Painter, R. H., Hofmann, T., and Shelton, E. (1977) Proc. Natl. Acad. Sci. USA, 74, 739-743.
Lei, K. J., Liu, T., Zon, G., Soravia, E., Liu, T. Y., and Goldman, N. D. (1985) J. Biol. Chem., 260, 13377-13383.
Syin, C., Gotschlich, E. C., and Liu, T. Y. (1986) J. Biol. Chem., 261, 5473-5479.
Volanakis, J. E., and Kaplan, M. H. (1971) Proc. Natl. Acad. Sci. USA, 136, 612-614.
Volanakis, J. E., and Wirtz, K. W. A. (1979) Nature, 281, 155-157.
Mold, C., Nakayama, S., Holzer, T. J., Gewurz, H., and Du Clos, T. W. (1981) J. Exp. Med., 154, 1703-1708.
Sui, S. F., Liu, Z., Li, W., Xiao, C. D., Wang, S. X., Gao, Q. F., and Zhou, Q. Z. (1996) FEBS Lett., 388, 103-111.
Potempa, L. A., Maldonado, B. A., Laurent, P., Zemel, E. S., and Gewurz, H. (1983) Mol. Immunol., 20, 1165-1175.
Potempa, L. A., Siegel, J. N., Fiedel, B. A., Potempa, R. T., and Gewurz, H. (1987) Mol. Immunol., 24, 531-541.
James, K., Hansen, B., and Gewurz, H. (1981) J. Immunol., 127, 2545-2552.
Fairbanks, T. R. (1984) PhD dissertation, Rush University, Chicago, Illinois, 60612.
Potempa, L. A., Gewurz, H., Harris, J. E., and Braun, D. P. (1986) Prot. Bio. Fluids, 34, 287-290.
Potempa, L. A., Zeller, J. M., Fiedel, B. A., Kinoshita, C. M., and Gewurz, H. (1988) Inflammation, 12, 391-405.
Potempa, L. A., Motie, M., Anderson, B., Klein, E., and Baurmeister, U. (1991) Clin. Materials, 11, 105-117.
Potempa, L. A., Motie, M., Wright, K. E., Crump, B. L., Radosevich, J. A., Sakai, N., Hua, L. G., Tanaka, K., Kojima, E., and Tsuboi, A. (1996) Exp. Hematol., 24, 258-264.
Fiedel, B. A., Simpson, R. M., and Gewurz, H. (1982) Immunology, 45, 439-447.
Shields, M. J. (1993) Immunol. Res., 12, 37-47.
Motie, M., Brockmerier, and Potempa, L. A. (1996) J. Immunol., 56, 4435-4441.
Diehl, E. E., Haines III, G. K., Radosevich, J. A., and Potempa, L. A. (2000) Am. J. Med. Sci., 319, 79-83.
Egenhofer, C., Alsdorff, K., Fehsel, K., and Kolb-Bachofen, V. (1993) Hepatology, 18, 1216-1223.
Samols, D., Macintyre, S. S., and Kushner, I. (1985) Biochem. J., 227, 759-765.
Rees, R. F., Gewurz, H., Siegel, J. N., Coon, J., and Potempa, L. A. (1988) Clin. Immunol. Immunopathol., 48, 95-107.
Kresl, J. J., Anderson, B., Radosevich, J. A., and Potempa, L. P. (1999) Tumor Biol., 20, 72-87.
Ballou, S. P., Buniel, J., and Macintyre, S. S. (1989) J. Immunol., 142, 2708-2715.
Zahedi, K., Tebo, J. M., Siripont, J., Klimo, F., and Mortensen, R. F. (1989) J. Immunol., 142, 2384-2392.
Sui, S. F., Sun, Y. T., and Mi, L. Z. (1999) Biophys. J., 76, 333-341.
Xiao, C. D., and Sui, S. F. (1999) Eur. Biophys. J., 28, 151-157.
Gotschlich, E. C., and Edelman, G. M. (1965) Proc. Natl. Acad. Sci. USA, 54, 558-566.
Myles, D. A. A., Rule, S. A., Delucas, L. J., Babu, Y. S., Xu, Y., Volanakis, J. E., Bugg, C. E., Bailey, S., and Greenhough, T. J. (1990) J. Mol. Biol., 216, 491-496.
Tannock, I. F., and Rotin, D. (1989) Cancer Res., 49, 4373-4384.
Cohen, M. S. (1994) Clin. Infect. Dis., 18, S170-179.
Stryer, L. (1965) J. Mol. Biol., 13, 482-495.
Wang, H. W., and Sui, S. F. (1999) J. Struct. Biol., 127, 283-286.
Wang, H. W., and Sui, S. F. (2001) J. Struct. Biol., 134, 46-55.
Eisenberg, M., Gresalfi, T., Riccio, T., and Mclaughlin, S. (1979) Biochemistry, 18, 5213-5223.
Kresl, J. J., Potempa, L. A., and Anderson, B. E. (1998) Biochem. Cell Biol., 30, 1415-1426.
Hack, C. E., Wolbin, G. J., Schalkwijk, C., Speijer, H., Hermens, W. T., and van den Bosch, H. (1997) Immunol. Today, 18, 111-115.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Y., Ji, SR., Wang, HW. et al. Study of the Spontaneous Dissociation of Rabbit C-Reactive Protein. Biochemistry (Moscow) 67, 1377–1382 (2002). https://doi.org/10.1023/A:1021862027061
Issue Date:
DOI: https://doi.org/10.1023/A:1021862027061