Abstract
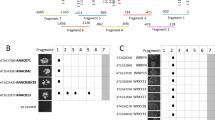
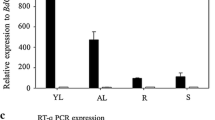
The peroxiredoxin antioxidant gene AtPER1 has been considered to be specifically expressed in the embryo and aleurone layer during maturation and desiccation stages of development, and in the mature seed, typically for late embryogenesis-abundant (lea) transcripts. In the abscisic acid-insensitive abi3-1 mutant, the AtPER1 transcript level is strongly reduced, suggesting ABI3 to be a prime regulator of AtPER1. We have studied the expression pattern and regulation of AtPER1 with a series of nine promoter::GUS constructs with deletions and/or mutations in putative regulatory elements. Arabidopsis lines harbouring these constructs revealed AtPER1 promoter activity in the endosperm, especially the chalazal cyst, already when the embryo is in the late globular stage, in the embryo from the late torpedo stage, and in distinct cells of unfertilized and fertilized ovules. Early expression seems to be dependent on a putative antioxidant-responsive promoter element (ARE), while from the bent cotyledon stage endosperm and embryo expression is dependent on an ABA-responsive element (ABRE) likely to bind ABI5. The shortest promoter fragment (113 bp), devoid of ARE, ABRE and without an intact RY/Sph element thought to bind ABI3 did not drive GUS expression. The AtPER1::GUSconstruct also revealed expression in cotyledons, meristems and stem branching points. In general, seed and vegetative expression coincided with the expression pattern of ABI3. In plants ectopically expressing ABI3, AtPER1::GUS expression was found in true leaves, and AtPER1 could be induced by exogenous ABA and oxidative stress (H2O2 and hydroquinone). ABI3-mediated oxidative stress induction was dependent on the presence of an intact ARE element.
Similar content being viewed by others
References
Aalen, R.B. 1999. Peroxiredoxin antioxidants in seed physiology. Seed Sci. Res. 9: 285–295.
Aalen, R.B., Opsahl-Ferstad, H.G., Linnestad, C. and Olsen, O.A. 1994. Transcripts encoding an oleosin and a dormancy-related protein are present in both the aleurone layer and the embryo of developing barley (Hordeum vulgare L.) seeds. Plant J. 5: 385–396.
Baumlein, H., Nagy, I., Villarroel, R., Inzé, D. and Wobus, U. 1992. Cis-analysis of a seed protein gene promoter – the conservative RY repeat CATGCATG within the legumin box is essential for tissue-specific expression of a legumin gene. Plant J. 2: 233–239.
Bechtold, N., Ellis, J. and Pelletier, G. 1993. In-planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C.R. Acad. Sci. Sér. Iii 316: 1194–1199.
Berger, F. 2003. Endosperm: the crossroad of seed development. Curr. Opin. Plant Biol. 6: 42–50.
Bobb, A.J., Chern, M.S. and Bustos, M.M. 1997. Conserved RY repeats mediate transactivation of seed-specific promoters by the developmental regulator PvALF. Nucl. Acids Res. 25: 641–647.
Carles, C., Bies-Etheve, N., Aspart, L., Leon-Kloosterziel, K.M., Koornneef, M., Echeverria, M. and Delseny, M. 2002. Regulation of Arabidopsis thaliana Em genes: role of ABI5. Plant J. 30: 373–383.
Clough, S.J. and Bent, A.F. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743.
Dickinson, C.D., Evans, R.P. and Nielsen, N.C. 1988. RY repeats are conserved in the 5-flanking regions of legume seed-protein genes. Nucl. Acids Res. 16: 371–371.
Erickson, A.M., Nevarea, Z., Gipp, J.J. and Mulcahy, R.T. 2002. Identification of a variant antioxidant response element in the promoter of the human glutamate-cysteine ligase modifier subunit gene: revision of the ARE consensus sequence. J. Biol. Chem. 277: 30730–30737.
Espelund, M., Stacy, R.A. and Jakobsen, K.S. 1990. A simple method for generating single-stranded DNA probes labeled to high activities. Nucl. Acids Res. 18: 6157–6158.
Espelund, M., Debedout, J.A., Outlaw, W.H. and Jakobsen, K.S. 1995. Environmental and hormonal regulation of barley lateembryogenesis-abundant (Lea) messenger RNAs is via different signal-transduction pathways. Plant Cell Environ. 18: 943–949.
Ezcurra, I., Ellerstrom, M., Wycliffe, P., Stalberg, K. and Rask, L. 1999. Interaction between composite elements in the napA promoter: both the B-box ABA-responsive complex and the RY/G complex are necessary for seed-specific expression. Plant Mol. Biol. 40: 699–709.
Ezcurra, I., Wycliffe, P., Nehlin, L., Ellerstrom, M. and Rask, L. 2000. Transactivation of the Brassica napus napin promoter by ABI3 requires interaction of the conserved B2 and B3 domains of ABI3 with different cis elements: B2 mediates activation through an ABRE, whereas B3 interacts with an RY/G-box. Plant J. 24: 57–66.
Finkelstein, R.R. and Lynch, T.J. 2000. The Arabidopsis abscisic acid response gene ABI5 encodes a basic leucine zipper transcription factor. Plant Cell 12: 599–609.
Galau, G.A., Hughes, D.W. and Dure, L. 1986. Abscisic acid induction of cloned cotton late embryogenesis-abundant (Lea) messenger RNAs. Plant Mol. Biol. 7: 155–170.
Goldmark, P.J., Curry, J., Morris, C.F. and Walker-Simmons, M.K. 1992. Cloning and expression of an embryo-specific mRNA upregulated in hydrated dormant seeds. Plant Mol. Biol. 19: 433–441.
Grini, P.E., Jürgens, G. and Hülskamp, M. 2002. Embryo and endosperm development is disrupted in the female gametophytic capulet mutants of Arabidopsis. Genetics 162: 1911–1925.
Guan, L.M., Zhao, J. and Scandalios, J.G. 2000. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22: 87–95.
Haslekås, C., Stacy, R.A., Nygaard, V., Culianez-Macia, F.A. and Aalen, R.B. 1998. The expression of a peroxiredoxin antioxidant gene, AtPER1, in Arabidopsis thaliana is seed-specific and related to dormancy. Plant Mol. Biol. 36: 833–845.
Haslekås, C., Viken, M. K., Grini, P.E., Nygaard, V., Nordgard, S.H., Meza, T.J. and Aalen, R.B. Seed 1-Cys peroxiredoxin antioxidants are not involved in dormancy, but contribute to inhibition of germination during stress. Plant Physiol., in press.
Jefferson, R.A. 1989. The GUS reporter gene system. Nature 342: 837–838.
Jefferson, R.A., Kavanagh, T.A. and Bevan, M.W. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907.
Karssen, C., Brinkhorst-van der Swan, D., Breekland, A. and Koornneef, M. 1983. Induction of dormancy during seed development by endogenous abscisic acid: studies on abscisic acid-deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 157: 158–165.
Kim, K., Kim, I.H., Lee, K.Y., Rhee, S.G. and Stadtman, E.R. 1988. The isolation and purification of a specific 'protector' protein which inhibits enzyme inactivation by a thiol/Fe(III)/O2 mixedfunction oxidation system. J. Biol. Chem. 263: 4704–4711.
Laux, T. and Jürgens, G. 1997. Embryogenesis: a new start in life. Plant Cell 9: 989–1000.
Lim, Y.S., Cha, M.K., Kim, H.K., Uhm, T.B., Park, J.W., Kim, K. and Kim, I.H. 1993. Removals of hydrogen peroxide and hydroxyl radical by thiol-specific antioxidant protein as a possible role in vivo. Biochem. Biophys. Res. Commun. 192: 273–280.
Meza, T.J., Kamfjord, D., Håkelien, A.M., Evans, I., Godager, L.H., Mandal, A., Jakobsen, K.S. and Aalen, R.B. 2001. The frequency of silencing in Arabidopsis thaliana varies highly between progeny of siblings and can be influenced by environmental factors. Transgenic Res. 10: 53–67.
Mowla, S.B., Thomson, J.A., Farrant, J.M. and Mundree, S.G. 2002. A novel stress-inducible antioxidant enzyme identified from the resurrection plant Xerophyta viscosa Baker. Planta 215: 716–726.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Nakamura, S., Lynch, T.J. and Finkelstein, R.R. 2001. Physical interactions between ABA response loci of Arabidopsis. Plant J. 26: 627–635.
Pak, J.H., Manevich, Y., Kim, H.S., Feinstein, S.I. and Fisher, A.B. 2002. An antisense oligonucleotide to 1-cys peroxiredoxin causes lipid peroxidation and apoptosis in lung epithelial cells. J. Biol. Chem. 277: 49927–49934.
Parcy, F. and Giraudat, J. 1997. Interactions between the ABI1 and the ectopically expressed ABI3 genes in controlling abscisic acid responses in Arabidopsis vegetative tissues. Plant J. 11: 693–702.
Parcy, F., Valon, C., Raynal, M., Gaubier-Comella, P., Delseny, M. and Giraudat, J. 1994. Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6: 1567–1582.
Pastori, G.M. and Foyer, C.H. 2002. Common components, networks, and pathways of cross-tolerance to stress. The central role of 'redox' and abscisic acid-mediated controls. Plant Physiol. 129: 460–468.
Polidoros, A.N. and Scandalios, J.G. 1999. Role of hydrogen peroxide and different classes of antioxidants in the regulation of catalase and glutathione S-transferase gene expression in maize (Zea mays L.). Physiol. Plant. 106: 112–120.
Rohde, A., Van Montagu, M. and Boerjan, W. 1999. The ABSCISIC ACID-INSENSITIVE 3 (ABI3) gene is expressed during vegetative quiescence processes in Arabidopsis. Plant Cell Environ. 22: 261–270.
Rohde, A., De Rycke, R., Beeckman, T., Engler, G., Van Montagu, M. and Boerjan, W. 2000. ABI3 affects plastid differentiation in dark-grown Arabidopsis seedlings. Plant Cell 12: 35–52.
Rohde, A., Prinsen, E., De Rycke, R., Engler, G., Van Montagu, M. and Boerjan, W. 2002. PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell 14: 1885–1901.
Rushmore, T.H., Morton, M.R. and Pickett, C.B. 1991. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 266: 11632–11639.
Sambrook, J., Fritsch, E. and Maniatis, T. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Plainview, NY.
Soderman, E.M., Brocard, I.M., Lynch, T.J. and Finkelstein, R.R. 2000. Regulation and function of the Arabidopsis ABAinsensitive 4 gene in seed and abscisic acid response signaling networks. Plant Physiol. 124: 1752–1765.
Stacy, R.A., Munthe, E., Steinum, T., Sharma, B. and Aalen, R.B. 1996. A peroxiredoxin antioxidant is encoded by a dormancyrelated gene, Per1, expressed during late development in the aleurone and embryo of barley grains. Plant Mol. Biol. 31: 1205–1216.
Suzuki, M., Kao, C.Y. and McCarty, D.R. 1997. The conserved B3 domain of VIVIPAROUS1 has a cooperative DNA binding activity. Plant Cell 9: 799–807.
Wasserman, W.W. and Fahl, W.E. 1997. Functional antioxidant responsive elements. Proc. Natl. Acad. Sci. USA 94: 5361–5366.
Wood, Z.A., Schroder, E., Harris, J.R. and Poole, L.B. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28: 32–40.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haslekås, C., Grini, P.E., Nordgard, S.H. et al. ABI3 mediates expression of the peroxiredoxin antioxidant AtPER1 gene and induction by oxidative stress. Plant Mol Biol 53, 313–326 (2003). https://doi.org/10.1023/B:PLAN.0000006937.21343.2a
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000006937.21343.2a