Abstract
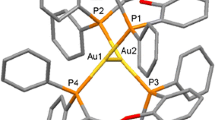
The structures of the tetragold(I) formamidinate cluster complexes, [Au4(ArNC(H)NAr)4], Ar=C6H4-4-OMe (1), C6H3-3,5-Cl (2), C6H4-4-Me (3), have been characterized by x-ray crystallography. The range of Au⋅⋅⋅Au distances is 2.8–3.0 Å. The angles at Au⋅⋅⋅Au⋅⋅⋅Au are acute and obtuse 70 and 109°, 88 and 91°, and 63 and 116° in 1, 2, and 3, respectively. The four gold atoms are located at the corner of a rhomboid with the formamidinate ligands bridged above and below the near plane of the four Au(I) atoms. The tetranuclear gold(I) complexes show a bright blue-green luminescence under UV light, with an emission at ∼490 nm and a weak emission at ∼530 nm in the solid state, at room temp and 77 K. The oxidation of the formamidinate cluster, 1, has been studied electrochemically in 0.1 M Bu4NPF6/CH2Cl2 at a Pt working electrode with different scan rates. Three waves were obtained, 0.75, 0.95, and 1.09V vs. Ag/AgCl at a scan rate of 500 mV/s, the three waves are reversible. The potentials are independent of the scan rate in the range 50 mV/s to 3 V/s. The current at the third wave is larger than those at the first two.
Similar content being viewed by others
References
(a) J. Barker and M. Kilner (1994). Coord. Chem. Rev. 133, 219-300 and references cited therein.
(b) S. Patai, The Chemistry of Amidines and Imidates, Vol. 1 (Wiley, New York, 1975).
(a) R. Clerac, F. A. Cotton, K. R. Dunbar, C. A. Murillo, and X. Wang (2001). Inorg. Chem. 40, 420-426.
(b) F. A. Cotton, C. Lin, and C. A. Murillo (2000). Inorg. Chem. 39, 4574-4578.
(c) F. A. Cotton, L. M. Daniels, C. A. Murillo, and P. Schooler (2000). J. Chem. Soc. Dalton Trans., 2007-2012.
(d) F. A. Cotton, L. M. Daniels, C. A. Murillo, and P. Schooler (2000). J. Chem. Soc. Dalton Trans., 2001-2005.
(e) F. A. Cotton, L. M. Daniels, J. H. Matonic, and C. A. Murillo (1997). Inorg. Chim. Acta 256, 277-282.
(a) H. H. Murray, R. G. Raptis, and J. P. Fackler, Jr. (1988). Inorg. Chem. 27, 26-33.
(b) R. G. Raptis and J. P. Fackler, Jr. (1988). Inorg. Chem. 27, 4179-4182.
R. G. Raptis, H. H. Murray, III, and J. P. Fackler, Jr. (1987). J. Chem. Soc., Chem. Commun. 10, 737-739.
A. A. Mohamed, J. M. López-de-Luzuriaga, and J. P. Fackler, Jr. (2003). J. Cluster Sci. 14, 61.
A. A. Mohamed, L. M. Perez, I. Kani, and J. P. Fackler, Jr. (2003). To be published.
H. Schmidbaur (ed.), Gold Progress in Chemistry, Biochemistry, and Technology (Wiley, West Sussex, England, 1999).
T. Ren, C. Lin, P. Amalberti, D. Macikenas, J. D. Protasiewicz, J. C. Baum, and T. L. Gibson (1998). Inorg. Chem. Commun. 1, 23-26.
S. J. Archibald, N. W. Alcock, D. H. Busch, and D. R. Whitcomb (1999). Inorg. Chem. 38, 5571-5578.
F. A. Cotton, X. Feng, M. Matusz, and R. Poli (1988). J. Am. Chem. Soc. 110, 7077-7083.
S. J. Archibald, N. W. Alcock, D. H. Busch, and D. R. Whitcomb (2000). J. Cluster Sci. 11, 261-283.
SMART V 4.043 Software for the CCD Detector System, Bruker Analytical X-ray Systems, Madison, WI, 1995.
SAINT V 4.035 Software for the CCD Detector System, Bruker Analytical X-ray Systems, Madison, WI, 1995.
SAINT V 4.035 Software for the CCD Detector System, Bruker Analytical X-ray Systems, Madison, WI, 1995.
R. H. Blessing (1995). SADABS. Program for absorption corrections using Siemens CCD based on the method of Robert Blessing, Acta Cryst. A 51, 33.
G. M. Scheldrick, SHELXS-97, Program for the Solution of Crystal Structure (University of Göttingen, Germany, 1997).
SHELXTL 5.03 (PC-Version), Program Library for Structure Solution and Molecular Graphics (Bruker Analytical X-ray Systems, Madison, WI, 1995).
R. Anulewicz, I. Wawer, T. M. Krygowski, F. Maennle, and H.-H. Limbach (1997). J. Am. Chem. Soc. 119, 12223-12230.
J. J. Guy, P. G. Jones, M. J. Mays, and G. M. Sheldrick (1977). J. Chem. Soc. Dalton Trans., 8-10.
J. Beck and J. Strahle (1986). Angew. Chem. Int. Ed. Engl. 25, 95.
B. Chiari, O. Piovesana, T. Tarantelli, and P. F. Zanazzi (1985). Inorg. Chem., 366-371.
G. Yang and R. Raptis (2003). Inorg. Chem. Act. 352, 98-104.
S. D. Bunge, Oliver Just, and William S. Rees, Jr. (2000). Angew. Chem. Int. Ed. 39, 3082-3084.
P. D. Harvey (1995). Inorg. Chem. 34, 2019-2024.
A. Vogler and H. Kunkley (1988). Chem. Phys. Lett. 150, 135-137.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mohamed, A.A., Abdou, H.E., Irwin, M.D. et al. Gold(I) Formamidinate Clusters: The Structure, Luminescence, and Electrochemistry of the Tetranuclear, Base-Free [Au4(ArNC(H)NAr)4]. Journal of Cluster Science 14, 253–266 (2003). https://doi.org/10.1023/B:JOCL.0000005062.20754.5f
Issue Date:
DOI: https://doi.org/10.1023/B:JOCL.0000005062.20754.5f