Abstract
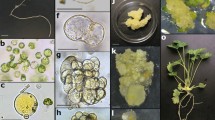
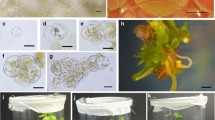
A protocol for rapid and efficient plant regeneration from protoplasts of red cabbage was developed by a novel nurse culture method. When the protoplasts of red cabbage were cultured in modified MS medium containing various combinations of BA, NAA and 2,4-D, they did not continue dividing due to browning. However, they successfully divided and formed micro-calli at a high efficiency when they were mixed and co-cultured with those of tuber mustard at a 1:1 ratio. The presence of tuber mustard protoplasts used as nurse cells was essential for sustainable divisions and colony formation of red cabbage protoplasts. Red cabbage-like plantlets were regenerated from these protoplast-derived calli at a frequency ranging from 33 to 56% in all the experiments where three cultivars of red cabbage were tested. Over 120 protoplast-derived cabbage plants were transferred to the greenhouse, and they showed no noticeable abnormalities in morphological features. Chromosome observation revealed that all of the plants examined had the normal chromosome number of cabbage (2n = 18), suggesting that no spontaneous fusion between the two species had occurred during protoplast culture.
Similar content being viewed by others
References
Chen LP, Zhang MF, Hirata Y, Cao JS & Chen ZJ (2001) Efficient plant regeneration from cotyledon-derived protoplasts of cytoplasmic male-sterile tuber mustard (Brassica juncea Coss. var. tumida Tsen et lee). Acta Phytophysiol. Sin. 27(5): 437–440
Fu YY, Jia SR & Lin Y (1985) Plant regeneration from mesophyll protoplast culture of cabbage (Brassica oleracea var ‘capitata’). Theor. Appl. Genet. 71: 495–499
Frearson EM, Power JB & Cocking EC (1973) The isolation, culture and regeneration of petunia leaf protoplasts. Dev. Biol. 33: 130–137
Glimelius K (1984) High growth rate and regeneration capacity of hypocotyl protoplasts in some Brassicaceae. Physiol. Plant. 61: 38–44
Hansen LN & Earle ED (1994) Regeneration of plant from protoplasts of rapid cycling Brassica oleracea L. Plant Cell Rep. 13: 335–339
Hansen LN, Ortiz R & Andersen SB (1999) Genetic analysis of protoplast regeneration ability in Brassica oleracea. Plant Cell Tiss. Org. Cult. 58: 127–132
Hu Q, Andersen SB & Hansen LN (1999) Plant regeneration capacity of mesophyll protoplasts from Brassica napus and related species. Plant Cell Tiss. Org. Cult. 59: 189–196
Jiang W, Cho MJ & Lemaux PG (1998) Improved callus quality and prolonged regenerability in model and recalcitrant barley (Hordeum vulgare L.) cultivars. Plant Biotechnol. 15(2): 63–69
Jourdan PS & Earle ED (1989) Genotypic variability in the frequency of plant regeneration from leaf protoplasts of four Brassica ssp. and Raphanus sativus. J. Am. Soc. Hort. Sci. 114: 343–349
Karamian R & Ebrahimzadeh H (2001) Plantlet regeneration from protoplast-derived embryogenic calli of Crocus cancellatus. Plant Cell Tiss. Org. Cult. 65: 115–121
Kik C & Zaal MACM (1993) Protoplast culture and regeneration from Brassica oleracea “rapid cycling” and other varieties. Plant Cell Tiss. Org. Cult. 35: 107–114
Kunitake H, Nakashima T, Mori K, Tanaka M & Mii M (1995) Plant regeneration from mesophyll protoplasts of lisianthus (Eustoma grandiflorum) by adding activated charcoal into protoplast culture medium. Plant Cell Tiss. Org. Cult. 43: 59–65
Kyozuka J, Hayashi Y & Shimamoto K (1987) High frequency plant regeneration from rice protoplasts by novel culture methods. Mol. Gen. Genet. 206: 408–412
Liu MC (1994) A novel method of plant regeneration from suspension culture protoplast of sugarcane. J. Plant Physiol. 143: 753–755
Matsuoka M & Sugimoto A (1997) Plant regeneration from protoplast-derived callus of sugarcane. Breed. Sci. 47: 301–305
Murashige T & Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15: 473–497
Walters TW & Earle ED (1990) A simple, versatile feeder layer system for Brassica oleracea protoplast culture. Plant Cell Rep. 9: 316–319
Wu JG, Li Z, Liu Y, Liu HL & Fu TD (1997) Cytogenetics and morphology of the pentaploid hybrid between Brassica napus and Orychophragmus violaceus and its progeny. Plant Breed. 116: 251–257
Zhao KN, Bittisnich DJ, Halloran GM & Whitecross MI (1995) Studies of cotyledon protoplast cultures from B. napus. B. campestris and B. oleracea. II. Callus formation and plant regeneration. Plant Cell Tiss. Org. Cult. 40: 73–84
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, LP., Zhang, MF., Xiao, QB. et al. Plant Regeneration from Hypocotyl Protoplasts of Red Cabbage (Brassica Oleracea) by Using Nurse Cultures. Plant Cell, Tissue and Organ Culture 77, 133–138 (2004). https://doi.org/10.1023/B:TICU.0000016811.29125.18
Issue Date:
DOI: https://doi.org/10.1023/B:TICU.0000016811.29125.18